mTOR signaling regulates Zika virus replication bidirectionally through autophagy and protein translation
Bin Liu, Yahui Zhang, and Hao Ren contributed equally to this study.
Abstract
Zika virus (ZIKV) reemerged in 2016 and attracted much more attention worldwide. To date, the limited knowledge of ZIKV interactions with host cells in the early stages of infection impedes the prevention of viral epidemics and the treatment of ZIKV disease. The mammalian target of rapamycin (mTOR) signaling pathway plays an essential role in the regulation of autophagy and protein synthesis during multiple viral infections. This study aimed to investigate the functional role of mTOR signaling in ZIKV replication in human umbilical vein endothelial cells. Immunoblotting demonstrated that ZIKV infection inhibited mTORC1 signaling, enhancing autophagy but obstructing protein translation. Drugs or siRNA for interfering with mTOR signaling molecules were utilized to demonstrate that AKT/TSC2/mTORC1 signaling was involved in ZIKV infection and that autophagy promoted ZIKV production, but viral protein expression was regulated by mTORC1 signaling. Moreover, confocal microscopy indicated a robust correlation between autophagy and viral RNA transcription. This study clarifies the dual functions of mTOR signaling during ZIKV infection and provides theoretical support for developing potential anti-ZIKV drugs based on mTOR signaling molecules and deeper insights to better understand the mechanism between ZIKV and host cells.
1 INTRODUCTION
Zika virus (ZIKV), isolated initially in Uganda in 1947, caused a reemerging epidemic outbreak worldwide in 2016. ZIKV is transmitted primarily through mosquito bites, but vertical and sexual transmissions are also documented in infected cases.1 The majority of ZIKV-infected individuals are asymptomatic or have febrile symptoms, but severe neurological symptoms can also manifest.2 Moreover, mother-to-fetus transmission results in the severe birth defect microcephaly, indicating that ZIKV can pass through placental and blood‒brain barriers.3 As a member of the Flaviviridae family, ZIKV is a single positive-stranded RNA virus with an 11 kb genome encoding three structural proteins, including envelope protein (E), capsid protein (C) and precursor membrane protein (prM), and seven nonstructural proteins (NSs).4 ZIKV entry is facilitated by E protein-interacting cell surface receptors, including the tyrosine-protein kinase receptor AXL and the tripartite motif containing 22, and then through endocytosis to infect a variety of cells.5-7 However, the interaction between ZIKV and host cells in the viral life cycle still needs to be further investigated.
Some studies have demonstrated that ZIKV infection could hinder host cell proliferation and trigger cell death in endothelial and neuronal cells.8 Mammalian target of rapamycin (mTOR) coordinates cell survival and proliferation with environmental stimuli; hence, it might be linked to ZIKV infection.9 mTOR, as a serine/threonine protein kinase in the PI3K protein family, forms two different complexes, mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2). mTORC1 contains three core components: mTOR, regulatory-associated protein of mTOR (Raptor) and mammalian lethal with Sec. 13 protein 8 (mLST8). In addition to mTOR and mLST8, mTORC2 contains another core component, rapamycin-insensitive companion of mTOR (Rictor). Rapamycin can interact with the FK506-binding protein12 (FKBP12) and then bind the FKBP12-rapamycin-binding (FRB) domain of mTOR, occupying the catalytic site and restraining the activation of downstream substrates.10 mTORC1 activity is suppressed directly by rapamycin, but mTORC2 is insensitive to rapamycin.11 mTORC1 promotes anabolism and inhibits catabolism for cell growth and division. Protein synthesis is enhanced by mTORC1 mainly through the phosphorylation of ribosomal S6 kinases (S6K) and eIF4E binding protein (4E-BP) for the initial stage of messenger RNA translation.12, 13 Autophagy, a cellular catabolism process and highly conserved defense response, plays important roles in multiple viral infections and replication under mTORC1 regulation. Autophagy initiation induces the formation of a double-membrane structure in the cytoplasm to encapsulate metabolites to develop autophagosomes. Mature autophagosomes fuse with lysosomes to form autophagolysosomes and ultimately degrade substrates.14 Inhibition of mTORC1 activity dephosphorylates S757 of UNC-51-like kinase 1 (ULK1) site but activates the Beclin-1 and VPS34 complex to induce the formation of autophagy precursor membrane structures in the cytoplasm.15 Esterified microtubule-associated protein 1 light chain 3 (LC3) II is anchored on the autophagosome membrane and promotes its elongation.16 In addition, mTORC1 can interrupt the nuclear translocation of transcription factor EB (TFEB) to suppress autophagy.17 mTORC2 mainly phosphorylates AKT at site S473 and activates PI3K/AKT signaling to regulate cell proliferation and survival.11
Previous studies have demonstrated that mTOR signaling is involved in viral infection and replication. Flaviviruses, including Japanese encephalitis virus (JEV), dengue virus (DENV), and white spot syndrome virus, were found to activate PI3K/AKT/mTORC1 signaling to increase viral protein expression.18, 19 Similar to other flaviviruses, ZIKV NS4A and NS4B proteins were proven to inhibit AKT/mTOR signaling during the infection of human fetal neural stem cells (fNSCs), causing defective neurogenesis and abnormal activation of autophagy.20 However, Sahoo et al. found that ZIKV infection activated mTOR signaling and suppressed autophagy to promote viral replication in neuronal and glial cells.21 In addition, previous work demonstrated that autophagy was triggered in ZIKV-infected human umbilical vein endothelial cells (HUVECs), but rapamycin, a mTOR inhibitor or autophagy inducer, did not affect viral protein expression or progeny production.22 These results revealed the complicated function of mTOR signaling in ZIKV infection, and further study is needed to explore the mechanism. Here, this study proved that ZIKV infection inactivated AKT/mTORC1 signaling to enhance autophagy but hindered protein translation, which promoted ZIKV production and downregulated viral protein expression. This study clarifies the dual functions of mTOR signaling in protein synthesis and autophagy induction during ZIKV infection to provide a deeper understanding of the interaction mechanism between ZIKV and host cells.
2 MATERIALS AND METHODS
2.1 Cells and virus
HUVECs and Vero cells were cultured at 37°C in complete Dulbecco's modified Eagle's medium containing 10% (v/v) heat-inactivated fetal bovine serum (Gibco BRL). C6/36 cells were cultured in RPMI-1640 (Gibco) supplemented with 10% FBS. ZIKV (strain SZ01, provided by Professor Cheng-Feng Qin from Beijing Institute of Microbiology and Epidemiology) was propagated in C6/36 cells, and the stock virus was stored at −80°C until use.
2.2 Antibodies and chemicals
Rapamycin, everolimus, temsirolimus, and SC79 were purchased from Selleck and dissolved in dimethyl sulfoxide (DMSO), DMSO, ethanol (EtOH) and DMSO, respectively. Polyclonal rabbit anti-LC3-II antibody was purchased from Sigma-Aldrich. Polyclonal rabbit anti-DENV NS1 was purchased from GeneTex (Irvine). Antibodies against AMPK, phospho-AMPK, AKT, phospho-AKT (S473), phospho-AKT (T308), TSC2, phospho-TSC2, mTOR, phospho-mTOR, S6K, phospho-S6K, 4E-BP1, phospho-4E-BP1, ULK1, phospho-ULK1 (S757), Beclin-1, Raptor, and Rictor were purchased from Cell Signaling Technology (Danvers). Monoclonal mouse anti-p62 and anti-glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibodies were obtained from Abcam and Abmart, respectively. Horseradish peroxidase (HRP)-conjugated secondary antibodies were purchased from Beyotime. The mTagRFP-mWasabi-LC3 reporter plasmid was kindly provided by Professor Lin Jian of Peking University. Small interfering RNA (siRNA) to knockdown mTOR, AMPK, TSC2, Rictor, and Raptor were constructed by Guangzhou RiboBio company.
2.3 Viral infection
Virus titers were determined by plaque assay and measured as the plaque-forming unit (PFU) per milliliter. HUVECs were infected with ZIKV for 1 h in serum-free opti-MEM. Then, the cells were washed with phosphate-buffered saline (PBS) and cultured in fresh complete media until they were harvested and examined. For mTOR inhibitors or the AKT activator SC79, cells were pretreated with different concentrations of drugs for 2 h before virus infection. For siRNA, cells were transfected with siRNA (100 nM) for 24 h before virus infection.
2.4 Plaque assay
Virus titers in the virus stock or the culture supernatant were determined by plaque assay. In brief, Vero cells were infected with serially diluted virus stock or supernatants from ZIKV-infected cells. After virus adsorption, the cells were washed with prewarmed PBS, followed by adding overlay medium containing 1.3% methylcellulose. The cells were incubated for 5–6 days at 37°C and then stained with 1% crystal violet. Plaques were counted, and the viral titers were calculated as PFU per milliliter.
2.5 Western blot
Cells were lysed with ice-cold RIPA lysis buffer (Beyotime) containing protease inhibitors. After centrifugation, protein concentrations were determined using the Bradford method (Beyotime). Proteins were then separated by 12.5% (w/v) SDS-polyacrylamide gel electrophoresis (PAGE) and transferred onto polyvinylidene fluoride membranes (Millipore) using a Trans-Blot apparatus (Bio-Rad). The proteins of interest were detected with specific primary antibodies and HRP-conjugated secondary antibodies. Immunoreactivity was visualized by enhanced chemiluminescence using SuperSignal West Pico chemiluminescent substrate (Thermo). Quantifications were performed using ImageJ software (National Institutes of Health) and adjusted with densitometry of the corresponding loading control (GAPDH).
2.6 Quantitative real-time polymerase chain reaction (qRT‒PCR)
Total RNA was extracted using TRIzol Reagent (Invitrogen) according to the manufacturer's instructions. One microgram of total RNA was reverse-transcribed using M-MLV reverse transcriptase (Promega) with random hexamers. Quantitative real-time PCRs were performed using a SYBR Green Master kit (TaKaRa) on a 7300Plus Real-Time PCR system (Thermo Fisher Scientific) and normalized to GAPDH. The sequences of specific primers are listed as follows:
ZIKV NS1, forward 5′-CGGCAATCAAGCCATCACT-3′
and reverse 5′-GCCTCGTCTCTTCTTCTCCTT-3′;
GAPDH, forward 5′-TGGGCTACACTGAGCACCAG-3′
and reverse 5’-AAGTGGTCGTTGAGGGCAAT-3’.
All reactions were carried out in triplicate on a StepOne Plus PCR system, and the data were analyzed by Prism software.
2.7 Confocal microscopy
For the detection of autophagosomes, cells were infected with lentivirus expressing mTagRFP-mWasabi-LC3. For the detection of autophagosomes, cells were infected with lentivirus expressing mTagRFP-mWasabi-LC3 as described before.22 The mTagRFP-mWasabi-LC3 reporter contains red and green fluorescence, and puncta that appears red and green, or yellow when merged, represent autophagosomes. After 48 h of lentiviral infection, the cells were infected with ZIKV for 1 h, and recombinant fluorescent LC3 was observed under a Zeiss or Leica SP8 confocal fluorescence microscope. The number of cells with punctate fluorescent LC3 localization relative to all positive fluorescent cells was counted (a minimum of 50 fluorescence-positive cells were counted in total for each condition).
2.8 Statistical analysis
Data are represented as the means ± standard deviations (SD) of independent experiments. The results were analyzed by Student's t test or one-way analysis of variance (ANOVA) using Prism software. Values were considered statistically significant when *p < 0.05, **p < 0.01, or ***p < 0.001.
3 RESULTS
3.1 ZIKV infection inhibited mTOR signaling to enhance autophagy and hinder protein translation
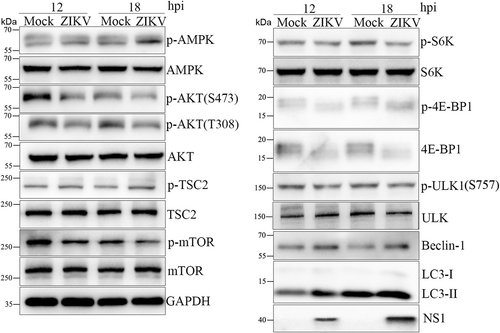
A robust correlation has been demonstrated between Flavivirus infection and mTOR signaling, which does not agree with the previous results that the mTOR inhibitor rapamycin did not affect ZIKV replication in HUVECs. This study first determined whether mTOR signaling was involved in ZIKV-infected HUVECs. HUVECs were infected with ZIKV at a multiplicity of infection (MOI) of 1 and harvested at 12 and 18 h post infection (hpi) for western blot to detect the expression of mTOR signaling molecules (Figure 1 and Supporting Information: Figure S3). The phosphorylation level of mTOR was decreased, and its upstream molecules TSC2 and AMPK were activated, while AKT was dephosphorylated, indicating that mTORC1 activity was inhibited in ZIKV-infected HUVECs. Meanwhile, S6K and 4E-BP1, the downstream targets of mTORC1, were phosphorylated to block protein translation. mTORC1 inhibited the phosphorylation of the ULK1 S757 site and increased the expression of Beclin-1 and LC3-II to enhance autophagy. Therefore, mTORC1 signaling was inhibited to induce autophagy and hinder protein synthesis in ZIKV-infected HUVECs.
3.2 mTOR knockdown also increased only viral RNA replication
A previous study found that rapamycin-induced autophagy but did not change viral protein expression or progeny production in ZIKV-infected HUVECs. Rapamycin can occupy the catalytic site of mTOR to inhibit mTORC1 activity.11 Rapamycin and its optimized derivatives, everolimus or temsirolimus, were utilized to determine whether the physical properties of rapamycin influenced ZIKV replication. The results indicated that they increased the intracellular viral RNA levels by approximately 2.5-fold but did not change viral protein NS1 expression or extracellular virus yields (Supporting Information: Figure S1), which suggested that rapamycin and its derivatives promote viral RNA transcription but not protein translation and excluded the influence of rapamycin's physical properties on ZIKV replication.
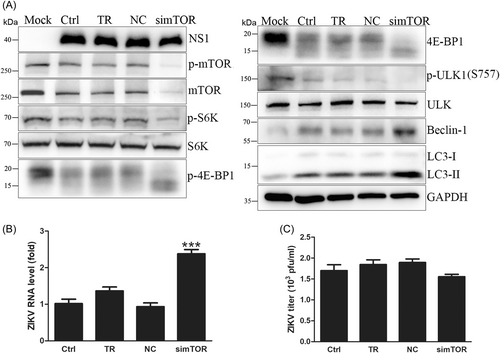
To further verify the effect of mTOR signaling on ZIKV replication, siRNA targeting mTOR was transfected into HUVECs for 48 h before infection, and mTOR protein expression was effectively knocked down by siRNA. Then, ZIKV was used to infect HUVECs at an MOI of 1, and the cells were collected at 18 hpi. Western blot showed that knockdown of mTOR increased intracellular viral RNA levels by approximately 2.4-fold but did not change NS1 expression or extracellular virus yields (Figure 2A–C), which suggested that knockdown of mTOR by siRNA promoted viral genome transcription, consistent with rapamycin and its derivatives. In addition, knockdown of mTOR by siRNA dephosphorylated S6K and 4E-BP1 to inhibit protein synthesis and increased the protein expression levels of Beclin-1 and LC3-II to enhance autophagy (Figure 2A and Supporting Information: Figure S4). Therefore, siRNA of mTOR proved again that inhibition of mTORC1 enhanced autophagy to promote ZIKV RNA replication, but protein expression and secretion of ZIKV progeny remained unchanged due to the suppression of protein translation.
3.3 TSC2 knockdown inhibited ZIKV replication
TSC2, a negative factor for mTORC1, contains a GTPase-activating protein domain to inactivate Rheb and inhibit mTORC1 activity.23 TSC2 absence usually results in abnormal mTORC1 activation. In addition, mTORC1 activity can be regulated by other upstream molecules: PRAS40 can be phosphorylated by AKT and inhibit mTORC1,24 and Raptor, the core component of mTORC1, can be phosphorylated by AMPK to inhibit mTORC1 activity directly.25 Given the complicated upstream regulatory signaling of mTORC1, the roles of TSC2 in mTORC1 activity and viral replication were investigated in ZIKV-infected HUVECs.
TSC2 was significantly knocked down by siRNA, and HUVECs were infected with ZIKV and harvested at 18 hpi. Knockdown of TSC2 largely reduced intracellular viral RNA levels by half, NS1 expression and extracellular virus yields (Figure 3A–C), indicating that TSC2 knockdown not only inhibited ZIKV genome transcription but also obstructed viral protein expression and secretion. Western blot analysis showed that TSC2 knockdown activated mTORC1 signaling to phosphorylate S6K and 4E-BP1 but reduced the protein expression levels of Beclin-1 and LC3-II to attenuate autophagy (Figure 3A and Supporting Information: Figure S5). In addition, mTORC1 inhibition by TSC2 knockdown did not induce the compensatory regulation of PRAS40 or Raptor, validating that TSC2 is the direct upstream negative regulator of mTORC1 in ZIKV-infected HUVECs. Autophagy attenuation by TSC2 knockdown hindered ZIKV genome transcription, although protein synthesis was enhanced by mTORC1 activation. Therefore, the inhibition of mTORC1 activity was directly regulated by TSC2 in ZIKV-infected HUVECs.

3.4 AKT activator inhibited ZIKV replication
AMPK and AKT, as upstream factors of TSC2, were regulated in ZIKV-infected HUVECs, both of which were involved in mTORC1 activity. The interaction between AMPK and mTORC1 depends on the cell energy supply.15, 25 The function of AMPK in viral replication in ZIKV-infected HUVECs was explored by siRNA to knockdown AMPK. The results showed that AMPK knockdown did not affect ZIKV replication (Supporting Information: Figure S2). Besides, AMPK knockdown did not change the phosphorylation of TSC2 and mTOR, but AKT was dephosphorylated (Supporting Information: Figure S2A), suggesting that AKT played an important role in ZIKV-infected HUVECs. AMPK and AKT antagonize mTORC1 activity through TSC2 phosphorylation,23 so AKT dephosphorylation may compensate for AMPK knockdown. In addition, AKT phosphorylates PRAS40 to attenuate the inhibition of mTORC1.24 The detailed mechanism of AKT in ZIKV-infected HUVECs is worth further investigation.
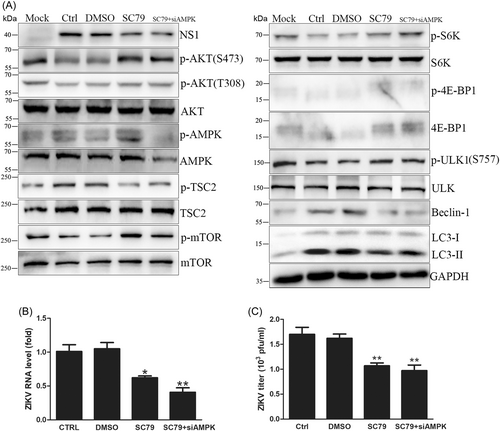
HUVECs were pretreated with the AKT activator SC79 for 2 h and infected with ZIKV for 18 h. AKT activation (with or without AMPK knockdown) significantly decreased intracellular viral RNA levels by one-third, NS1 expression and extracellular virus yields (Figure 4A–C), indicating that AKT activation inhibited viral replication. Western blot analysis showed that AMPK phosphorylation and mTORC1 activity were increased by SC79 without AMPK knockdown, and AMPK knockdown combined with SC79 also enhanced mTORC1 activity (Figure 4A and Supporting Information: Figure 6). Therefore, ZIKV infection inhibited mTORC1 activity mainly through AKT/TSC2 signaling. In addition, SC79 increased the phosphorylation of S6K and 4E-BP1, while Beclin-1 and LC3-II protein expression levels decreased (Figure 4A). Attenuated autophagy blocked viral RNA transcription even though protein synthesis was promoted. Hence, AKT activation by SC79 inhibits ZIKV replication.
3.5 Knockdown of the mTORC1 core component Raptor promoted ZIKV replication
Different complexes formed by mTOR perform different functions in cellular metabolism: mTORC1 promotes anabolism and inhibits catabolism for cell growth and division, but mTORC2 mainly regulates cell proliferation and survival. To identify the specific functions of the two complexes, siRNA was used to knock down Raptor, a mTORC1 core component that facilitates the ability of mTOR to bind substrates accurately26 or directly interacts with AMPK to inhibit mTORC1 activity,24 to investigate the effect of mTORC1 on viral replication in ZIKV-infected HUVECs.
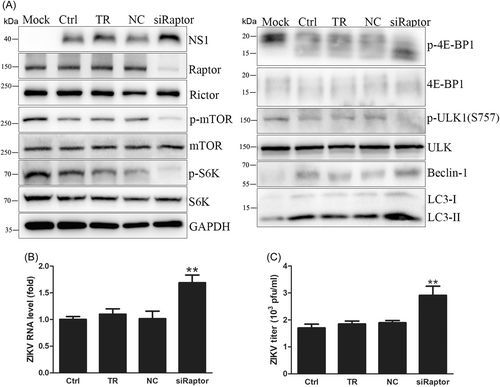
Raptor knockdown by siRNA was confirmed by western blot. Raptor knockdown increased intracellular viral RNA levels by approximately 1.6-fold, NS1 expression, and extracellular virus yields (Figure 5A–C), proving that Raptor knockdown promoted ZIKV replication. In addition, Raptor knockdown dephosphorylated mTOR to inhibit mTORC1 activity so that S6K and 4E-BP1 were dephosphorylated to inhibit protein synthesis, and Beclin-1 and LC3-II expression levels were increased to enhance autophagy, consistent with the results of mTOR knockdown and its inhibitors (Figure 5A and Supporting Information: Figure S7). However, mTOR knockdown and its inhibitors only augmented viral RNA transcription, but Raptor knockdown also promoted viral protein translation and progeny release. Further analysis found that mTOR knockdown and its inhibitors resulted in a higher increase in intracellular viral RNA levels than Raptor knockdown (Figure 6D). Considering that Raptor assists mTORC1 in binding substrates and that mTOR knockdown and its inhibitors directly inhibit mTORC1 activity, inhibition of mTORC1 activity by Raptor knockdown was milder than that achieved by mTOR knockdown and its inhibitors. Besides, mTOR knockdown and its inhibitors induced a higher degree of autophagy and the intracellular viral RNA levels than Raptor knockdown (Figure 7B). It is more likely that mTOR knockdown and its inhibitors promoted viral genome transcription because of the higher enhancement of autophagy than Raptor knockdown, as did the variation trends in protein synthesis inhibition. Lower autophagy enhancement by Raptor knockdown promoted ZIKV genome transcription, while milder inhibition of protein synthesis was so limited that even its reserved capacity still guaranteed viral protein expression. In contrast, protein synthesis inhibition by mTOR knockdown and its inhibitors was so tremendous that viral protein expression was disturbed. Hence, Raptor knockdown promoted ZIKV replication, while mTOR knockdown and its inhibitors only enhanced viral RNA levels.
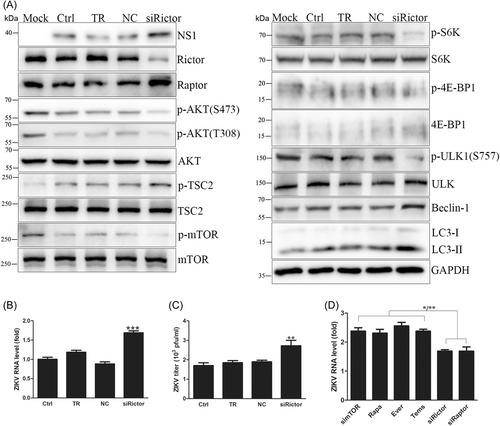
3.6 Knockdown of the mTORC2 core component Rictor benefited ZIKV replication
mTORC2 works in cell survival and proliferation mainly through phosphorylation of AKT at the S473 site to activate PI3K/AKT signaling.27 Rictor, the mTORC2 core component, was knocked down to investigate the function of mTORC2 in viral replication in ZIKV-infected HUVECs.
Rictor expression was knocked down effectively by siRNA. Rictor knockdown increased intracellular viral RNA levels by approximately 1.7-fold, NS1 expression and extracellular virus yields (Figure 6A–C). Rictor knockdown promoted ZIKV genome transcription, protein translation and progeny release, which was identical to Raptor knockdown. Western blot results showed that Rictor knockdown dephosphorylated AKT and indirectly disturbed mTORC1 activity, leading to protein synthesis inhibition and autophagy enhancement (Figure 6A and Supporting Information: Figure S8). These results proved that mTORC2 regulated ZIKV replication in an AKT-dependent manner. Compared with mTOR knockdown and its inhibitors, Rictor knockdown caused a lower increase in intracellular viral RNA levels and was the same as Raptor knockdown (Figure 6D), indicating a lower autophagy enhancement of Rictor knockdown. In addition, slight inhibition of protein synthesis by Rictor knockdown did not impede viral protein translation. Therefore, Rictor knockdown promoted ZIKV replication by AKT dephosphorylation to inhibit mTORC1 activity.
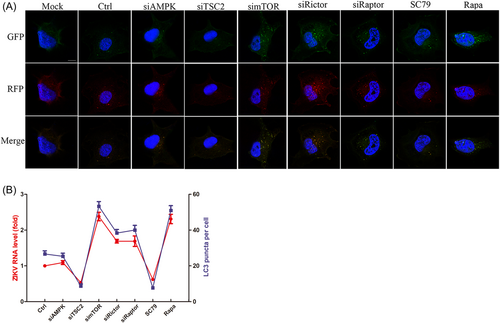
3.7 ZIKV-induced autophagy was regulated by mTOR signaling disruption
mTORC1 inhibition dephosphorylated ULK1 at the S757 site to activate the VPS34 complex, which promoted the formation of precursor membrane structures.28 Subsequent esterification of LC3-I to LC3-II elongates membrane structures to form autophagosomes for autophagy initiation.16 To visualize the process of ZIKV-induced autophagy regulated by mTOR signaling, the mTagRFP-mWasabi-LC3 reporter plasmid was utilized to detect autophagosomes.
HUVECs were infected with lentivirus expressing mTagRFP-mWasabi-LC3 for 48 h and then infected with ZIKV combined with siRNA or drugs to disturb mTOR signaling. AMPK knockdown did not change the amount of fluorescent LC3 puncta compared with that in infected cells. TSC2 knockdown and AKT activation decreased fluorescent LC3 puncta, while mTOR and its inhibitor, Raptor, and Rictor knockdown noticeably increased the number of fluorescent LC3 puncta, indicating the differential regulation of mTOR signaling molecules on ZIKV-induced autophagy (Figure 7A). Further analysis found that the degree of autophagy induction caused by mTOR signaling disturbance was positively correlated with the intracellular viral RNA levels based on the qRT‒PCR results by Pearson's two-tailed t-test (Figure 7B). Previous studies have found that ZIKV infection hijacks membrane-structured vesicles, which are often derived from autophagy-triggered ER stress, to accomplish genome replication and progeny assembly. Therefore, enhanced autophagy induced by ZIKV infection in HUVECs benefits viral replication.
4 DISCUSSION
mTOR signaling has been proven to have a robust correlation with some viral infections, such as white spot syndrome virus, JEV and DENV.18, 19 This study found that ZIKV infection dephosphorylated AKT but phosphorylated AMPK to inhibit mTORC1 activity, enhancing autophagy but disturbing protein synthesis (Figure 8). Conversely, some viruses activate AKT/mTORC1 signaling to promote protein synthesis and benefit viral replication, such as Middle East respiratory syndrome coronavirus and some flaviviruses.18, 29 The latest genetic analysis has speculated that AKT/mTOR signaling can be activated and involved in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.30 Moreover, ZIKV infection activated mTORC1 and mTORC2 to inhibit autophagy and promote viral replication in human neuronal precursor cells or glial cells, in contrast to HUVECs.21 In contrast, ZIKV NS4A and NS4B inhibited AKT/mTOR signaling to induce autophagy dysfunction and fNSCs,20 consistent with mTOR signaling regulation in ZIKV-infected HUVECs. Therefore, multiple studies have indicated the complicated regulation of mTOR signaling and autophagy during different viral infections, which resulted from the unique genomes of different viruses or the selective expression profiles of different cells. Therefore, the specific regulatory mechanism of mTOR signaling in ZIKV-infected HUVECs needs further research.
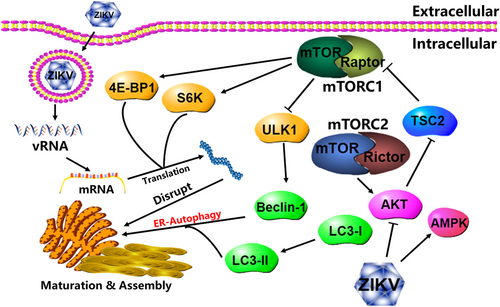
Previous studies found that the autophagy inducers torin1 or rapamycin increased intracellular viral RNA levels and extracellular virus production when ZIKV infected primary fibroblasts or human fibroblasts.31, 32 However, the results showed that mTOR knockdown or its inhibitors induced autophagy and only facilitated viral genome transcription but did not change viral protein expression or progeny release. Autophagy induction is correlated with the mobilization and conformation of intracellular membrane structures, such as endocytosis or exocytosis, which have been proven to be regulated by GTPases.33 GTPases have been demonstrated to be autophagy-associated genes (ATGs) and participate in vesicle budding, transport, fusion, and packaging.34 Flaviviruses induce autophagy in the early stage of viral infection to inhibit apoptosis and innate immunity and promote lipid consumption for viral replication.35 Similarly, ZIKV infection of skin fibroblasts or human neural precursor cells resulted in endoplasmic reticulum (ER) rearrangement and induced the formation of vesicle packets (VPs) in the cytoplasm, which encapsulated viral particles and was used as the platform for viral RNA replication, so-called viral pockets.32, 36, 37 Therefore, ZIKV infection inhibited mTORC1 activity to enhance autophagy, which accelerated VP production to provide independent space for viral RNA replication and mobilize intracellular membrane structures for progeny secretion. However, protein synthesis was hindered by dephosphorylation of S6K and 4E-BP1, so viral protein expression and progeny release were limited. Autophagic vesicles were observed during SARS-CoV-2 or swine coronavirus infection and utilized for viral invasion and viral replication.38, 39 Surprisingly, knockdown of Atg5 or Atg7 during West Nile virus (WNV) infection inhibited autophagy but did not affect viral replication, suggesting that WNV replication was not regulated by the autophagy process and was different from that of other flaviviruses.40, 41
ZIKV infection of HUVECs dephosphorylated AKT but phosphorylated AMPK to inhibit mTORC1. To determine the upstream regulation of mTORC1 in ZIKV-infected HUVECs, siRNA targeting AMPK and TSC2 and the AKT activator SC79 were used to observe mTOR signaling alterations and ZIKV replication. TSC2 knockdown activated mTORC1 signaling to attenuate autophagy and promote protein translation, and ZIKV replication was inhibited. Theoretically, AMPK knockdown could increase mTORC1 activity and inhibit ZIKV replication, but mTOR signaling and ZIKV replication were not changed with AKT dephosphorylation, which was regarded as a compensatory reaction for AMPK knockdown to maintain mTORC1 activity. In contrast to AMPK knockdown, AKT activation significantly activated mTOR signaling and inhibited ZIKV replication with or without AMPK knockdown, consistent with TSC2 knockdown attenuating autophagy and enhancing protein synthesis. Taken together, it was concluded that mTORC1 activity is inhibited mainly through AKT/TSC2 signaling in ZIKV-infected HUVECs to inhibit protein synthesis but enhance autophagy, corresponding with signaling regulation in ZIKV-infected fNSCs.20 Given the robust correlation of RNA transcription and autophagy inducement (Figure 7B) and the previous result that autophagy benefited ZIKV replication,22 TSC2 knockdown and AKT activation attenuated autophagy to decrease RNA level and then inhibit viral replication in ZIKV-infected HUVECs. ZIKV infection of human neuronal precursor cells and glial cells discriminately activated AKT/TSC2/mTORC1 signaling and inhibited autophagy to promote viral replication.21 These results demonstrated that AKT/TSC2/mTORC1 signaling but not AMPK participated in most viral infections. However, AMPK can bypass mTORC1 and directly work on the ULK1 complex to regulate autophagy under insufficient cellular energy. A recent study found that SARS-CoV-2 inhibited mTORC1 activity by activating AMPK to produce an immunosuppressive effect in the upper respiratory tract,42 indicating the importance of AMPK in mTOR signaling regulation.
mTORC1 regulates cellular metabolism for cell growth and division to affect viral infection, and mTORC2 mainly depends on the activation of PI3K/AKT signaling to regulate cell survival.9 The poxvirus F17 protein binds to Rictor or Raptor to disturb the crosstalk of mTORC1 and mTORC2 and inhibits the antiviral effect of mTOR during its own infection.43 In this study, knockdown of Raptor or Rictor, the core components of mTORC1 and mTORC2, respectively, both promoted ZIKV replication. Autophagy enhancement provided a membrane platform for viral replication and increased intracellular viral RNA levels. However, while dephosphorylation of S6K and 4E-BP1 obstructed posttranscriptional translation, intracellular viral protein expression and extracellular virus production were still elevated. Given the indirect work on mTORC1 of Rictor and Raptor knockdown, which activated AKT signaling or facilitated interaction with substrate, respectively, mTOR knockdown and its inhibitors worked on mTOR molecules directly and markedly. Raptor or Rictor knockdown inhibited mTORC1 activity less than mTOR knockdown or its inhibitors, and the reserved capacity of protein synthesis still guaranteed viral protein expression to benefit ZIKV replication, which can be reflected from qRT-PCR results and confocal observations of autophagy. Because Raptor or Rictor knockdown resulted in a lower increase in intracellular viral RNA levels than mTOR knockdown or its inhibitors (Figure 6D) and there was a positive correlation of RNA transcription and autophagy inducement (Figure 7B), Raptor or Rictor knockdown induced a lower enhancement of autophagy to promote viral genome transcription. Peterson et al. found that mTORC1 inhibition dephosphorylated S6K and 4E-BP1, but protein phosphatase 2A was phosphorylated to guarantee a certain capacity for protein synthesis.44 Therefore, it is speculated that the necessary protein synthesis ability was conserved even if Raptor or Rictor knockdown inhibited mTORC1 activity. The qRT-PCR results suggested that intracellular viral RNA levels were positively associated with autophagy induction but negatively related to protein synthesis. Therefore, the effect on ZIKV replication by disturbing mTOR signaling in HUVECs depends on the inhibition degree of mTORC1 activity.
ZIKV-induced autophagy in HUVECs is closely linked with mTORC1 activity and ZIKV replication. mTOR signaling mainly regulates the initiation stage of autophagy, and LC3-II, a marker for autophagy initiation, works on the accumulation of autophagosomes. Based on the visualization of the mTagRFP-mWasabi-LC3 reporter, the number of fluorescent LC3 puncta was positively correlated with intracellular viral RNA levels, demonstrating that autophagy promoted ZIKV genome transcription. Previous studies found that the NS4A and NS4B proteins of flaviviruses triggered ER stress and developed a “chamber” for viral replication.45-47 In addition, coexpressing ZIKV NS4A and NS4B in HeLa cells or fNSCs increased LC3-II accumulation and even inhibited AKT/mTORC1 signaling to enhance autophagy, suggesting the important functions of ZIKV NS4A and NS4B in autophagy induction.20 It can be speculated that NS4A and NS4B play important roles in autophagy induction in ZIKV-infected HUVECs through the regulation of mTOR signaling.
In conclusion, ZIKV infection of HUVECs inhibited the AKT/TSC2/mTORC1 signaling pathway to enhance autophagy and hinder protein translation. Autophagy promoted ZIKV replication, but viral protein expression was regulated by mTORC1 activity. This study clarified the dual roles of mTOR signaling in protein synthesis and autophagy induction during ZIKV infection on ZIKV replication, providing potential targets based on mTOR signaling to develop anti-ZIKV drugs. To date, ZIKV infection is still a threat and challenge to global public health, and it is of great significance to explore the mechanism of ZIKV-host cell interaction and develop effective methods for virus infection control and disease treatment.
AUTHOR CONTRIBUTIONS
Zhongtian Qi, Zhaoling Qin, and Ping Zhao designed experiments. Bin Liu, Yahui Zhang, Hao Ren, Qiufeng Yao, Jianbo Ba, Jie Luan performed experiments and analyzed data. Bin Liu, Yahui Zhang, Hao Ren wrote the manuscript. Zhongtian Qi, Zhaoling Qin, and Ping Zhao reviewed and edited the manuscript. All authors approved the manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




