HPV-related cervical diseases: Alteration of vaginal microbiotas and promising potential for diagnosis
Abstract
Vaginal microbiota is closely associated with women's health, however, the correlation between HPV-related cervical disease (HRCD) and vaginal microbiota is still obscure. In this study, patients with HRCD (n = 98) and healthy controls (n = 58) in Hangzhou were recruited, and their vaginal microbiota were collected and analyzed. The composition of the vaginal microbial community was explored, and a disease classification model was developed by random forest algorithm. The results suggested that the diversity of vaginal microbiota was significantly higher in HRCDs than that in healthy controls (p < 0.05). Firmicutes is the dominant phylum in vaginal microbiota, and Lactobacillus was identified as the most altered genus between two groups (p < 0.01). Kyoto Encyclopedia of Genes and Genomes analysis suggested the difference in vaginal microbial community functions between two groups. Furthermore, we identified 10 biomarkers as the optimal marker sets for the random forest model and found a higher probability of disease values in HRCD group in discovery cohort (p < 0.0001), with an area under the receiver operating characteristic curve reaching 89.7% (95% confidence interval: 78.3%–100%). We further validated the model in both validation and independent diagnosis cohorts, confirming its accuracy in the prediction of HRCD. In conclusion, this study revealed the community composition of vaginal microbiota in HRCDs and successfully constructed a diagnostic model for HRCD.
1 INTRODUCTION
Human papillomavirus (HPV) is a double-stranded DNA virus which mainly infects the skin and mucous membranes. Some warts on the surface of the skin and mucous, such as genital warts, are attributed to HPV infection. In addition, HPV infection is responsible for the initial progression of many human tumors, such as cervical, anal, penile, and head and neck cancers.1 It has been reported that 4.5% of tumor cases diagnosed are attributed to HPV infection worldwide in 2012, while this proportion reached 8.6% in women.1, 2 HPV infection is no doubt a major health risk in women. Strikingly, the economic and social burden caused by HPV infection is second only to human immunodeficiency virus (HIV) in sexually transmitted diseases.3
Most women will be infected with HPV one or more times during their life cycle, and most infections are transient and can be cleared by the immune system within 2 years, while the rest will develop as persistent infection and eventually transform into cervical intraepithelial neoplasia (CIN) or even cervical cancer.4 HPV infection leads to a variety of female cervical diseases, including cervical cancer, CIN, genital warts and etc., in addition, HPV infection is also closely associated with cervical inflammation.5, 6 Cervical cancer, as a serious cervical lesion, is the most common malignancy in the female reproductive system, almost all cervical cancer cases are caused by HPV infection.7 Cervical cancer contributes to approximately 600 000 newly diagnosed cancer cases, and over 300 000 deaths in women each year worldwide.8
Microbiota participates in some fundamental biological processes, including immunity, nutrition, and metabolism, and is an integral microecological system in the human body.9 With the increasing interests in intestinal microbiota, the significance of microbiota in health has been more extensively described, as well as the vaginal microbiota, the microorganisms colonizing the vagina. The female vagina hosts a large number of microorganisms, accounting for approximately 9% of the total human microbial population,10 and the vaginal microbiota is a dynamic microecology which could be affected by age, pregnancy status, contraceptive use, menstruation, and sexual behavior,9, 11 for example, the abundance of Lactobacillus will increase with age in puberty girls, and various microbial communities composition is observed at different gynecological life cycle in women.12 The vaginal microbiota plays an important role in maintaining vaginal homeostasis, and the stability of the microbiota prevents the colonization and infection of some external pathogenic microorganisms. Normal vaginal flora is usually dominated by Lactobacillus, such as Lactobacillus iners, Lactobacillus crispatus, Lactobacillus gasseri, Lactobacillus jensenii, and Lactobacillus gallinarum,11, 13-15 also some distributions of a wide variety of other microbial species.16 However, it seems that a Lactobacillus-dominated vaginal microecology is not essential for vaginal health, since some obligate anaerobic bacteria was investigated to dominate the vaginal microbiota, the commonness is that these microbes could generate lactic acid, suggesting the important role of lactic acid in maintaining a healthy vaginal microenvironment.11 Healthy female vaginal microbial communities are usually characterized by low biodiversity. Increased vaginal microbial diversity usually closely correlates with female reproductive system diseases, for instance, fluctuation in the vaginal microbial community induced by bacterial vaginosis leads to a reduced abundance of Lactobacillus,17 accompanied by the increase in the microbial diversity.18 As illustrated previously, some compounds derived by vaginal microbiota, including lactic acid and H2O2, exhibit direct inhibitory effects on HIV, furthermore, kidnaped virus by lectins on the bacterial surface could be another mechanism for vaginal microbiota to prevent the viral infection, as well as stimulating host immune responses.19 Similarly, a close relationship between vaginal microbiota composition and HPV infection has also been well documented, women with L. iners-dominated or high diversity vaginal microbiota are more likely to be HPV positive,20 while those with L. gasseri-dominated vaginal microbiota show faster HPV clearance.21 In addition, the severity of CIN was positively correlated with the diversity of vaginal microbiota.22 Given the tight association between vaginal microbiota and female reproductive system diseases, several reports have established models for disease prediction. Lee et al.23 identified bacterial markers between CIN 1− and CIN 2+ patients, which could effectively distinguish the severity of CIN. Another report by an independent group constructed a model based on the community functional analysis of cervical microbiota to successfully predict the occurrence and progression of CIN.24 Previous investigation also noted that early HPV infection may be responsible for the decrease of Shuttleworthia, Prevotella, and Lactobacillus, and the increase of Dispar, Streptococcus, and Faecalibacterium, and thus, such alternations in community composition could be potential indicators for HPV infections and related diseases.25
In the present study, we prospectively collected 156 vaginal microbiota samples from Hangzhou, Zhejiang, China, including 98 patients with HPV-related cervical disease (HRCD) and 58 healthy controls. Here, HRCD is defined as the disease usually caused by HPV infection, including CIN and cervical cancer, while for the others, no medical treatment is recommended, such as warts are excluded, despite they might be induced by HPV infection. The samples were randomly divided into a discovery cohort, validation cohort, and further, public data were downloaded and served as an independent diagnosis cohort. In the discovery cohort, we analyzed the vaginal microbiome of healthy controls and HRCD patients and constructed an HRCD classification model using a random forest algorithm. We then validated the diagnosis efficacy of the HRCD classification model in the validation cohort and independent diagnosis cohort. Our data revealed the composition characteristics of vaginal microbial community in patients with HRCD and provided some experimental and theoretical basis for the development of diagnostic methods for HRCD using microbial markers of the vaginal microbiota.
2 METHODS AND MATERIALS
2.1 Sample collection and study design
One hundred and two patients with HRCD and 59 healthy controls in Hangzhou Zhejiang, China, were recruited during a routine gynecological visit at Zhejiang Cancer Hospital, China.
Inclusion and exclusion criteria: Participants diagnosed with HRCD or healthy controls aged from 18 to 75 were included. Those who were (a) diagnosed with combined other malignant tumors, (b) myocardial infarction or other cardiovascular diseases, (c) organ transplants or immune diseases, (d) severe mental disorders, (e) drug abuse, and (f) gestation and perinatal stages were excluded.
Vaginal swab was used to collect the vaginal microbiota as described previously with minimal modifications.26 The swabs were placed in a sterile tube and stored at −80°C for further use. After removing five samples, due to missing information or pollution, the remained samples (98 HRCDs and 58 healthy controls) were divided into a discovery cohort (50 HRCDs and 37 healthy controls) and a validation cohort (48 HRCDs and 21 healthy controls). We further obtained vaginal microbiota data of CIN or cervical cancer patients from a published report27 (n = 143, after removing the poor quality samples), which served as the independent diagnosis cohort in our study, the abundance and annotation of the OTUs were provided (Supporting Information: Table S1).
2.2 DNA extraction and 16S rRNA amplicon sequencing
The DNA was extracted using PowerSoil® DNA Isolation Kit (MoBio) according to the manufacturer's instructions. Extracted DNA was then subjected to 1% agarose gels and subsequently quantified using NanoDrop 2000 spectrophotometer. The final DNA concentration was diluted to 1 ng/μl. The bacterial 16S rRNA V3 region was amplified using universal primer pair, and the PCR reactions were conducted using Phusion® High-Fidelity PCR Master Mix (New England Biolabs). The PCR products were next determined by 2% agarose gel, and DNA ranging from 400 to 450 bp was collected for further use. The libraries were constructed using TruSeq® DNA PCR-Free Sample Preparation Kit (Illumina) in accordance with the manufacturer's protocol, and then sequenced on an Illumina MiSeq platform.
2.3 OTU cluster and annotation
The obtained 300 bp paired-end reads were assembled using FLASH (V1.2.11), and the quality filtering was carried out using QIIME (V1.9.1). After removing the chimera sequences using UCHIME, the sequences were analyzed with Uparse software (Uparse V8.1.1861), and those with ≥97% similarity were assigned as one operational taxonomic unit (OTU). SILVA reference database was employed for the OTU annotation.
2.4 Bacterial diversity analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG)-based functional annotation
Alpha diversity indices including observed-species, Chao1, Shannon, Simpson and ACE, and Beta diversity on both weighted and unweighted UniFrac were calculated with QIIME (V1.9.1). UniFrac is a widely used tool for the analysis of the difference in microbial communities.28 Principal component analysis and principal coordinate analysis (PCoA) were conducted to visualize the community difference among groups with dimensionality reduction algorithms using FactoMineR and WGCNA packages, respectively, in R software (Version 3.2.2). Linear discriminant analysis effect size (LEfSe) was analyzed to screen biomarkers with a statistical difference using an online tool.29 Microbial community function profile was predicted using Tax4Fun software.30
2.5 Identification of microbial OTU markers
Significantly different OTUs between two groups in the discovery cohort were tested by Student's t-test. The filtered OTUs in the random forest classification model were then subjected to fivefold cross-validation. An optimal set of 10 biomarkers was determined according to the fivefold cross-validation and further used to test the model, to obtain the probability of disease for discovery, validation, and independent diagnosis cohorts. The data of the independent diagnosis cohort was obtained from a previous publication.27 The receiver operating characteristic (ROC) curve was generated using the pROC package, and the area under the ROC (AUC) curves was used to evaluate the classification model.
3 RESULTS
3.1 Increase of microbial diversity in HRCD
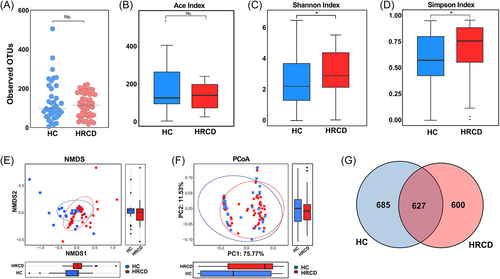
As mentioned above, the vaginal microbiota of healthy females is usually dominated by Lactobacillus, which implies a low microbial diversity. In the current study, we collected 161 vaginal microbiota samples from Hangzhou, Zhejiang, China, 3 were excluded owing to missing information, another 2 were excluded owing to the pollution (final sample size n = 156), detailed clinical information of the participants is included in Supporting Information: Tables S2 and S3. The recruited samples were randomly divided into in discovery cohort (n = 87), validation cohort (n = 69). We also downloaded the data from a published report (18 were excluded owing to poor sequencing quality and annotation, final sample size n = 143), which is served as the independent diagnosis cohort. The study design and cohorts set were displayed in Figure 1. The OTUs of vaginal microbiota in the discovery cohort, including HRCDs (n = 50) and healthy controls (n = 37), were first analyzed, and no significant difference in OTU number existed between two groups (Figure 2A). Alpha diversity including Ace, Shannon, and Simpson indices was calculated to gain an insight into the biodiversity of the vaginal microbiota. Higher Shannon and Simpson indices were observed in the HRCD group comparing to the healthy controls (p < 0.05), while no significant difference in terms of the Ace index (Figure 2B–D). The difference in composition of vaginal microbiota between two groups was evaluated by nonmetric multidimensional scaling analysis and the PCoA, however, according to our results, we failed to effectively distinguish between those two groups (Figure 2E,F). We further displayed the correlation between the two groups using a Venn diagram, there are 627 out of the total 1912 OTUs shared by two groups, 600 and 685 unique OTUs were possessed by HRCD and healthy control groups, respectively (Figure 2G). These results indicated that there were differences between HRCD and healthy control groups in the vaginal microbial biodiversity and community composition, further analysis is required for more details.
3.2 Vaginal microbial community composition in HRCD
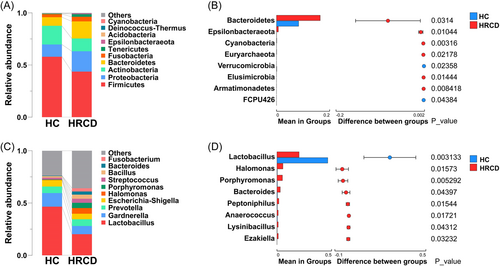
With knowing the increased vaginal microbial biodiversity in HRCDs, we next analyzed the composition of vaginal microbiota based on the abundance. As shown in Figure 3A, the Firmicutes was the most abundant phylum, accounting for 43.7% and 57.9% of the total OTUs in HRCDs and healthy controls, respectively, followed by Proteobacteria, Actinobacteria, and Bacteroidetes, these four most abundant phyla occupied over 90% of total OTUs. Six phyla, Bacteroidetes, Epsilonbacteraeota, Cyanobacteria, Euryarchaeota, Elusimicrobia, and Armatimonadetes were found enriched in HRCDs, while two phyla, Verrucomicrobia and FCPU426 were enriched in healthy controls (p < 0.05, Figure 3B). We further investigated the relative abundance of vaginal microbiota in the genus level, as indicated in Figure 3C, Lactobacilus was the most abundant genus in both groups, followed by Gardnerella, Prevotella, and Escherichia–Shigella, besides, 38 genera were found significantly different between the two groups, only 8 genera, including Lactobacilus, DNF00809, Akkermansia, and Gryllotalpicola were observed enriched in healthy controls, whereas, the others were enriched in HRCDs (Figure 3D and Supporting Information: Table S4, p < 0.05). Also, the composition and abundance of vaginal microbiota were analyzed at class, order, family, and species levels. At the class level, Bacilli was identified as the most abundant population, and Gammaproteobacteria and Bacteroidia were listed as the second and the third, respectively, and Bacilli and Verrucomicrobiae were more abundant in healthy controls (p < 0.05, Supporting Information: Figure S1A,B). At order level, Lactobacillales, Bacteroidales, and Bifidobacteriales were the most abundant populations. Five orders including Lactobacillales, Verrucomicrobiales, and IMCC26256 were identified as increased in healthy controls, and 11 orders including Bacteroidales, Clostridiales, Bacillales, and Oceanospirillales were found as increased in HRCDs (p < 0.05, Supporting Information: Figure S2A,B). There were 7 families enriched in healthy controls at the family level, including Lactobacillaceae, Eggerthellaceae, and Akkermansiaceae, the rest 13 families were enriched in HRCDs (p < 0.05, Supporting Information: Figure S3A,B). The top 3 dominant species in both groups were L. iners, Gardnerella vaginalis, and Escherichia coli (Supporting Information: Figure S4A), 7 species were enriched in healthy controls, while 10 were enriched in HRCDs (p < 0.05, Supporting Information: Figure S4B).
3.3 Key microbes and microbial functions related to HRCD
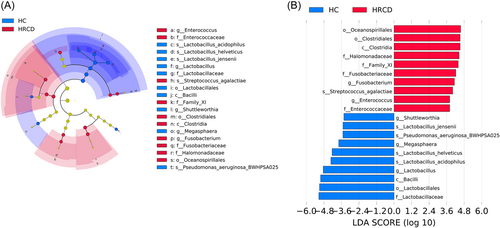
To evaluate the crucial microbial biomarkers between HRCD and healthy control groups, LEfSe was conducted. As indicated in Figure 4A,B, one class Clostridia, two orders Oceanospirillales and Clostridiales, four families including Halomonadaceae, Family_X, Fusobacteriaceae, and Enterococcaceae, two genera Fusobacterium and Enterococcus, and one species Streptococcus agalactiae were significantly enriched in HRCDs. The class Bacilli was found enriched in healthy controls, as well as the order Lactobacillales and the family Lactobacillaceae. Besides, three genera Shuttleworthia, Megasphaera, and Lactobacillus were also increased in healthy controls. Four species were noted enriched in healthy controls, including L. jensenii, Pseudomonas aeruginosa BWHPSA025, Lactobacillus helveticus, and Lactobacillus acidophilus.
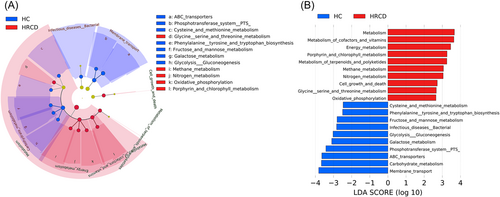
In addition, we predicted the vaginal microbial community function profiles based on KEGG pathway and KEGG orthology. Several pathways were found enriched in the healthy control group, including membrane transport, carbohydrate metabolism, ABC transporters, phosphotransferase system, cysteine and methionine metabolism, phenylalanine tyrosine and tryptophan biosynthesis, fructose and mannose metabolism, galactose metabolism, for the other pathways enriched in HRCD group, they were metabolism of cofactors and vitamins, porphyrin and chlorophyll metabolism, metabolism of terpenoids and polyketides, methane metabolism, nitrogen metabolism, glycine serine and threonine metabolism, oxidative phosphorylation (Figure 5A,B).
3.4 Diagnostic potential of HRCD based on the vaginal microbial biomarkers
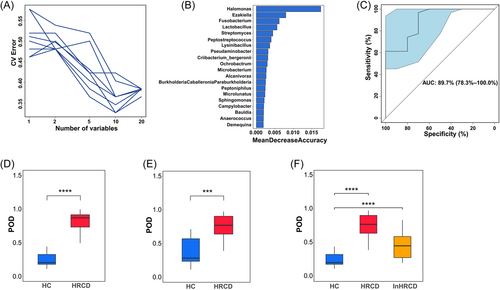
Microbial biomarkers are useful tools in predicting some human diseases.31, 32 It is intriguing to establish the prediction model to distinguish between HRCDs and healthy controls based on vaginal microbial biomarkers, which may serve as a promising approach for clinic diagnosis of HRCD. We, therefore, constructed a random forest classification model using samples in the discovery cohort. We first tested the random forest model by using different numbers of OTUs and identified 10 OTUs as the optimal marker set when assessed by a fivefold cross-validation (Figure 6A). The top 20 OTUs and MeanDecreaseAccuracy were shown in Figure 6B. The area under the ROC curve (AUC) reached 89.7% (95% confidence interval [CI]: 78.3%–100%) (Figure 6C). As indicated in Figure 6D, the probability of disease (POD) value in the HRCD group is significantly higher than that in the healthy control group (p < 0.0001). Subsequently, we tested the model in the validation cohort, and the result showed higher POD in HRCDs (p < 0.001, Figure 6E). Furthermore, HRCDs from the validation cohort and independent cohort were combined with healthy controls from the discovery cohort to test the accuracy of the model. The result showed the POD values in both HRCD groups are significantly higher than the healthy controls (p < 0.0001, Figure 6F). The results further validate the effectiveness of the predicting model for HRCD. Additionally, we regrouped the samples with different sample size (see the grouping information in Supporting Information: Table S5), discovery cohort (n = 48, 30%), and validation cohort (n = 108, 70%), for the construction of the classifier model. As expected, similar results were obtained with 81.9% AUC (95% CI: 73.0%–90.9%) (see Supporting Information: Figure S5).
4 DISCUSSION
Vaginal microbiota is a complex and dynamic microecosystem that fluctuates continuously during the menstrual cycle and throughout the female life cycle. A growing body of study suggests the tight association between the vaginal microbiota community and female diseases. However, few studies reveal the difference between the community composition of female vaginal microbiota with HRCD and the healthy, and further its worth as diagnostic biomarkers for HRCD.
In the current study, we collected vaginal microbiota samples from women with HRCD and healthy women in Hangzhou and investigated the vaginal microbiota community composition by 16S rRNA amplicon sequencing. We identified biomarkers for the HRCD diagnosis by using a random forest classification model. These results suggested that biomarkers of the vaginal microbiota have the potential to be served as a noninvasive diagnostic tool for HRCD.
Our results demonstrated that the biodiversity of the vaginal microbiota significantly increased in the HRCD group when compared to healthy controls, which is in agreement with some previous investigations. The healthy vaginal microbiota is usually dominated by Lactobacillus or some other anaerobic bacteria, which maintain their dominance by secreting substances deleterious to other microbes, resulting in a stable microenvironment.11 Correspondingly, the increase in vaginal microbiota diversity implies, to some extent, the loss of dominance of, for example, Lactobacillus. Our results suggested the greatest abundance of Firmicutes in both groups compared to other phyla, and its abundance in the HRCD group is lower than that in healthy controls. While for Bacteroidetes, its abundance is higher in HRCDs. Some recent investigations noted the association of the variation of Bacteroidetes abundance with some female diseases, for instance, women who succeed in in vitro fertilization possess a lower Bacteroidetes abundance in vaginal microbiota than those who failed.33 In addition, a higher abundance of Bacteroidetes was observed in the vagina of women with bacterial vaginosis,18 as well as those with bacterial vaginosis relapse.34 However, the alteration of Bacteroidetes abundance in patients with CIN is less clear.23, 25 At genus level, we noticed 38 genera were significantly varied between the two groups, and Lactobacillus is the genus of the most notable difference. Lactobacillus produces lactic acid and some other antimicrobial compounds, that is, H2O2 and bacteriocins,35 to prevent the infestation of other pathogenic microorganisms. A negative correlation between Lactobacillus abundance and the severity of CIN has been described.22 Recent investigation also highlighted that the culture supernatant of Lactobacillus exhibits its inhibitory effect on the expression of autophagy genes and HPV E6 in HeLa cells.36 In addition, women ingesting Lactobacillus as a daily supplement have a higher capacity to clear HPV.37 Mutually, HPV infection, no doubt, plays a role in educating the vaginal microbiota. A recent study by Lebeau et al.38 uncovered the regulatory role of HPV infection in Lactobacilli survival. In this study, HPV E7 oncoprotein was found to suppress the expression of host defense peptides by interacting with the core proteins in the NF-κB and Wnt/β-catenin pathways, the decreased defense peptides impair the survival of Lactobacillus which lives by using these defense peptides as amino acid sources, and subsequently rebuild the balance. These findings, combined with our results, suggest that increased diversity and decreased abundance of Lactobacillus in vaginal microbiota are strongly associated with the occurrence of HRCD. Different from the alteration of Lactobacillus, the abundance of Halomonas is dramatically increased in HRCDs, which is identified as one dominant population in women's vagina,39 previous report has noticed a decrease of Halomonas in patients with a missed miscarriage, suggesting its potential contribution in vaginal health.40 Furthermore, Halomonas is identified as the dominant genus in vaginal microbiota in healthy women and those with CIN3 and cervical cancer.24 However, little is known about the role of Halomonas in maintaining vaginal microbiota homeostasis. We further regrouped the cohorts, with 48 samples employed for the predictive model construction. These two models performed well in classifying the healthy and the disease, despite having different sets of optimal biomarkers. Both Lactobacillus and Halomonas were identified as the top 20 OTUs in our classification models. Ezakiella is less reported in previous research, whereas, according to a report published in 2017, a new member of Ezakiella genus, Ezakiella massiliensis, was isolated from the vagina of a woman who had sex with another woman with bacterial vaginosis.41 In our result, the genus Ezakiella was much higher in abundance in HRCDs (data not shown), which implicated the potential involvement in vaginal microbiota dysbiosis. However, when using a smaller sample size to construct the model, the genus Ezakiella was not included as the top 20 OTUs. There were several other genera being great importance in disease prediction, such as Streptomyces, Peptostreptococcus, Bauldia, since their coexistence in both models. Genus Peptostreptococcus might benefit the development of cervical cancer.42 Additionally, when vaginal homeostasis is disrupted, for example, the replacement of Lactobacillus population by other microbes, favors the growth of Peptostreptococcus anaerobius.43 While for Streptomyces and Bauldia, there are few information available with regard to vaginal microbiota.
Based on our analysis of the functional profile of vaginal microbiota community, several pathways were found differently enriched in two groups. Besides, recent investigation has demonstrated that vaginal microbiome of a female with endometriosis/adenomyosis show an upregulation in the metabolism of cofactors and vitamins,44 excess vitamin metabolism by vaginal microbiota might lead to vitamin deficiency for the host, as some previous study disclosed the association of vitamin D and bacterial vaginosis,45 on the other hand, sufficient vitamin supplements are negatively associated the risk of cervical cancer.46 Terpenoids derived from microbiota are mainly the metabolites of the host's bile acids, some Firmicutes are found take participate in the dehydration of bile acids, to form deoxycholic acid and lithocholic acid, which is harmful to human cells.47 In this study, the metabolism of terpenoids and polyketides was upregulated in HRCDs, however, it seems unlikely for the vagina to contact with bile acids, which may merit further attention. It has been reported that the porphyrin and chlorophyll metabolism pathway is inhibited by HPV infection,25 which may explain why lower hemoglobin levels in some cervical cancer patients.48 Microbial function involved in methane metabolism seems to elevate with the gestational age at delivery,49 despite the limited understanding, we might speculate a disturbance in the female reproductive tract during the pregnancy. We noticed the function enrichment involved in nitrogen metabolism, as described previously that vaginal microbiota with high diversity (e.g., women in reproductive and menopausal phases) trends to have higher capacity in the metabolism of nitrogen and sulfur.12 Usually, healthy vaginal dominant Lactobacillus mainly use glycogen as the energy source, while for some bacterial vaginosis-related microbiotas, amino acids and nitrogen are the preferred primary energy source.50 Thus, the ectopic nitrogen metabolism of the vaginal might indicate the failure of Lactobacillus dominance. Since Lactobacillus is the most abundant genus, it is no doubt that the enrichment of carbohydrate metabolism, including glycolysis/gluconeogenesis, fructose and mannose metabolism, and galactose metabolism, is in healthy controls. Another one of the most significantly enriched microbial function in healthy controls is the ABC transporter, a pathway involved in many basic biological functions, which also includes antibiotic resistance,51 while some strains associated with this pathway may also be involved in high-risk HPV infection and CIN.52 Another enriched pathway in healthy controls, the phosphotransferase system, is positively associated with high-risk HPV infection and bacterial vaginosis.52 Although it is difficult for us to figure out the correlation between vaginal microbial community functions and the occurrence of HRCD currently, alterations in these functions may imply the importance of vaginal microbiota in the pathogenesis of HRCD.
In conclusion, our study depicted the difference between the vaginal microbial community in HRCD and healthy controls, and further validate the potential of vaginal microbial markers to serve as the diagnostic tool for HRCD.
AUTHOR CONTRIBUTIONS
Xiaoxian Xu and Hanmei Lou conceived and designed the experiments. Xiaoxian Xu, Huiting Rao, Yichen Wang, Lingqin Zhao, Jie Xing, and Xiaojuan Lv collected the samples. Xiaoji Fan, Xiangwei Pang, and Jin Tao performed sequencing and analyzed the data. Xiaoxian Xu, Huiting Rao, Lingqin Zhao, Jie Xing, Xiaojuan Lv, and Xiaojing Zhang performed the clinic tests for the participants. Xiaoxian Xu, Huiting Rao, and Xiaoji Fan drafted the manuscript. Tingzhang Wang, Hanmei Lou, and Jianhua Qian reviewed the manuscript. Hanmei Lou and Jianhua Qian supervised the research. All the authors read and approved the final manuscript.
ACKNOWLEDGMENTS
The study was supported by the Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (No. 2018KY306, No. 2021KY083, and No. 2020KY478); Key project of Medical and health science and technology Plan of Zhejiang Province (WKJ-ZJ-2020); Zhejiang Province Public Welfare Technology Application Social Development Project (LGF19H160009); GCIG CCRN Research Grant.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Local Institutional Ethics Committee (IBR-2021-42). All participants provided written informed consent with knowing the study's purpose.
Open Research
DATA AVAILABILITY STATEMENT
Sequences obtained were deposited to National Microbiology Data Center (NMDC) (https://nmdc.cn/) with accession number NMDC10018205 (https://nmdc.cn/resource/genomics/project/detail/NMDC10018205). Besides, the data that support the findings of this study are openly available in NCBI Sequence Read Archive at https://doi.org/10.1186/s12879-020-05324-9, Ref. [27].




