Pathogenesis and histological changes of nephropathy associated with COVID-19
Abstract
Coronavirus disease 2019 (COVID-19) can cause damage to multiple organ, not only to the lungs, but also to the kidneys. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) can cause acute and chronic kidney disease through direct viral infection, indirect injury, and vaccination-related injury. Like lung injury, kidney injury is also an important aspect affecting the severity and prognosis of SARS-CoV-2. This article summarizes the pathogenesis, pathological manifestations, and clinical features of SARS-CoV-2 direct or indirect renal injury. Including direct injury, indirect injury, special comorbidities (receiving kidney transplantation and chronic kidney disease), and vaccine-related renal injury, and exploring the possible therapeutic effect of anti-SARS-CoV-2 therapy on renal injury. The purpose is to provide reference for understanding COVID-19-related renal injury, guiding clinical and pathological diagnosis and treatment, and evaluating prognosis.
Abbreviations
-
- ACE2
-
- Angiotensin I Converting Enzyme 2
-
- ADAMTS13
-
- a disintegrin and metalloproteinase with thrombospondin motifs 13
-
- AKI
-
- acute kidney injury
-
- ANCA
-
- antineutrophil cytoplasmic antibodies
-
- APOL1
-
- Apolipoprotein L1
-
- APTT
-
- Activated Partial Thromboplastin Time
-
- ARDS
-
- acute respiratory distress syndrome
-
- CKD
-
- chronic kidney disease
-
- COVID-19
-
- coronavirus disease 2019
-
- INF-γ
-
- interferon-gamma
-
- INR
-
- international normalized ratio
-
- IRF3
-
- interferon regulatory factor 3
-
- KDIGO
-
- Kidney Disease Improving Global Outcomes
-
- MAC
-
- membrane attack complex
-
- MAPK
-
- mitogen activated protein kinase
-
- MCP-1
-
- monocyte chemoattractant protein-1
-
- MIP-1
-
- macrophage inflammatory protein-1
-
- PEEP
-
- positive end-expiratory pressure
-
- RT-PCR
-
- reverse transcription polymerase chain reaction
-
- SARS-CoV-2
-
- severe acute respiratory syndrome coronavirus-2
-
- STAT1
-
- signal transducer and activator of transcription-1
-
- TMPRSS2
-
- Transmembrane Protease, Serine 2
-
- TNF-a
-
- tumor necrosis factor alpha
-
- VWF
-
- Von Willebrand factor
1 INTRODUCTION
The outbreak of coronavirus disease 2019 (COVID-19) has rapidly evolved into a global pandemic. Kidneys are frequently affected by COVID-19 and >40% of patients have abnormal proteinuria at hospital admission.1 Acute kidney injury (AKI) is a common and serious complication.2 The reported incidence of AKI in COVID-19 ranges from 0.5% to 56.9% in various case series.1, 3-10 There are many mechanisms underlying COVID-19-associated kidney damage, of which the most significant mechanism is the direct attack of SARS-CoV-2 on the innate renal cells. COVID-19 patients with hypertension, diabetes, or kidney transplantation may also have other mechanisms underlying the kidney injury. Renal biopsy from COVID-19 patients exhibits a wide range of histopathologic diagnoses, the most common of which is collapsing glomerulopathy. Collapsing glomerulopathy includes many rare diseases, such as myoglobin cast nephropathy, thrombotic microangiopathy, proliferative glomerulonephritis with monoclonal Immunoglobulin G (IgG) deposits, and hemoglobin cast nephropathy.11-14 The renal histological changes in COVID-19 patients differ with age, sex, and race. The mechanisms underlying kidney injury, disease severity, and renal histological changes vary between COVID-19 patients. Therefore, we summarized the pathophysiological mechanism and renal histological changes in kidney injury in different populations of COVID-19 patients.
2 INCIDENCE AND RISK FACTORS OF COVID-19-ASSOCIATED KIDNEY INJURY
SARS-COV-2 is mainly transmitted by droplets and direct contact. Most patients have dyspnea as the main clinical manifestation. However, some patients develop multi-system and organ damage, including to the heart, liver, and nervous system. SARS-COV-2 may also cause kidney damage, and the incidence rate of kidney damage in COVID-19 is approximately 10.6%–45%. A meta-analysis of 49 692 patients reported an incidence of AKI of 10.6% in all COVID-19 patients and of 22.1% in severely affected COVID-19 patients2; however, the data included in the meta-analysis were mainly collected from China. A study from South Africa showed that COVID-19 accounted for 33.9% of hospitalized patients.15 Studies from Europe, the United States, and the United Kingdom reported that AKI occurred in 28.6% of hospitalized patients with COVID-19,16, 17 and the incidence was as high as 40%–45% in patients with severe COVID-19.9, 18, 19 The mortality rate was as high as 71%–82%.20, 21 The incidence of AKI in COVID-19 patients with pre-existing glomerular disease was 2.7-fold higher than that in patients without previous renal disease (38% vs. 14%, respectively).22 A study by Flythe et al. reported that COVID-19 patients with chronic kidney disease (CKD) had a mortality rate of nearly 50%, which is 1.5-fold higher than that in patients without CKD.23 The STOP-COVID study showed that the 28-day mortality was 1.41- and 1.28-fold higher in CKD patients receiving replacement therapy and non-dialysis than non-CKD patients.24 AKI occurs in 44%–63% of kidney transplant patients, with a mortality rate of 10%–21%.25 Elderly patients with renal transplantation or undergoing renal replacement therapy have a significantly increased risk of death, possibly due to the higher prevalence of comorbidities in CKD patients. At the same time, the risk of death in kidney transplant patients is 1.28-fold higher than that in dialysis patients, which may be related to long-term immunosuppressive therapy.26 A study reported that the possible risk factors for AKI in COVID-19 patients were young or advanced age, male sex, obesity, comorbid CKD,2, 27-31 hypertension, diabetes, kidney transplantation, cardiovascular disease, severe COVID-19, use of vasopressors, and mechanical ventilation. COVID-9 patients with the aforementioned demographic characteristics and comorbidities have a 1.4- to 10.7-fold increased risk of developing AKI2, 8, 15, 30-34 (Table 1). In addition, SOFA score is closely related to AKI and treatment strategy. The study found that Higher non renal SOFA score is a risk factor for kidney injury in patients with COVID-19.35 Patients with a SOFA score > 8 at the time of AKI diagnosis had a 5.2 times greater risk of requiring renal replacement therapy.36 A study from India found that patients with COVID-19 combined with mucormycosis infection would aggravate kidney injury and increase the risk of patient death.37
| Factor risk | Risk of AKI (OR) | 95% CI | Reference |
|---|---|---|---|
| Patient factors | |||
| Age ≥ 60years | 1.3 | 2.9−4.2 | 2 |
| 5~15years>Age ≥ 60years | 2.7 | 1.7−4.6 | 30 |
| Males | 1.4 | 1.1−1.7 | 15 |
| African | 3.1 | 1.5−6.4 | 31 |
| Obesity | 2.7 | 1.0−7.3 | 32 |
| CKD | 1.6 | 1.4−1.9 | 33 |
| Kidney transplant | 5.9 | 1.3−26.9 | 34 |
| Diabetes | 1.8 | 1.5−2.1 | 8 |
| Hypertension | 1.3 | 1.0−1.5 | 8 |
| Cardiovascular disease | 1.5 | 1.2−1.8 | 8 |
| Disease factors | |||
| Severe COVID-19 | 6.6 | 2.8−15.4 | 2 |
| Mechanical ventilation | 10.7 | 6.8−16.7 | 8 |
| Vasoactive medication | 14.4 | 4.1−49.8 | 34 |
- Abbreviations: AKI, acute kidney injury; CI, confidence interval; OR, odd ratio.
3 MECHANISMS UNDERLYING KIDNEY DAMAGE CAUSED BY COVID-19
SARS-COV-2 can cause kidney damage via direct and indirect mechanisms. Some susceptibility factors play an important role in SARS-COV-2-associated kidney damage,38 such as being black and vitamin D deficiency. Apolipoprotein L1 (APOL1) is a high-risk genotype present in approximately 13% of African-Americans; this population has a 30-fold higher risk of kidney disease, particularly podocytopathies, compared to the general population.39 Studies have found that kidney damage caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is often accompanied by mutations in the APOL1 gene, which may be explained by the mitochondrial dysfunction and impaired glomerular podocyte membrane integrity, leading to podocyte destruction.40, 41 In addition, vitamin D modulates the immune system by upregulating T cell expression, thereby reducing the severity of cytokine storm.42 Black people are at higher risk of vitamin D deficiency due to factors such as skin pigmentation, have higher mutations in genes encoding vitamin d-binding proteins, and obesity, leading to an increased risk of severe complications in blacks infected with SARS-COV-2. The mechanism underlying kidney injury after SARS-COV-2 infection are described below (Figure 1).
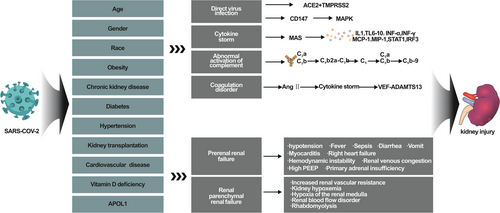
4 MECHANISMS RELATED TO INDIRECT DAMAGE
Hypotension is an important mechanism of prerenal kidney injury in COVID-19 patients, and may be caused by fever, sepsis, dehydration caused by diarrhea and vomiting, myocarditis, right heart failure leading to hemodynamic instability, and renal venous congestion.43-46 Furthermore, prerenal kidney injury may also be caused by the high positive end-expiratory pressure levels required for mechanical ventilation.47 Other studies have found that primary adrenal insufficiency induced by SARS-COV-2 infection may also cause hypotension and consequently kidney damage.48, 49 Acute respiratory distress syndrome (ARDS), carbon dioxide retention, and increased renal vascular resistance leading to renal hypoxemia and renal medullary hypoxia are important causes of renal AKI. Studies have found that ARDS caused by SARS-COV-2 infection predisposes to renal blood flow disorders compared to ARDS due to other causes.50 Myositis and rhabdomyolysis may occur in 19%–33% of SARS-COV-2 patients,51-53 Nephrotoxic drugs and iodine-containing contrast media are also important causes of AKI in COVID-19 patients.54
5 DIRECT DAMAGE MECHANISMS
5.1 Direct virus infection
It is unclear whether SARS-COV-2 directly damages the renal parenchymal cells. During the early stage of the COVID-19 pandemic, some studies demonstrated the presence of virus particles in renal parenchymal cells through electron microscopy and suggested that SARS-COV-2 is a cytopathic virus that enters the host cells through the membrane protein Angiotensin I Converting Enzyme 2 (ACE2).55, 56 The high expression of ACE2 in proximal tubular epithelial cells is a potential target of kidney injury.57 In the embryonic kidney, ACE2 and Transmembrane Protease, Serine 2 (TMPRSS2) co-localize in the proximal tubule.58 TMPRSS2 and ACE2 act as coreceptors to activate the spike protein on the surface of SARS-CoV-2, so that the membrane fuses into the host cell and participates in SARS-CoV-2-associated kidney damage.59 However, it is also believed that the expression sites of TMPRSS2 and ACE2 are proximal renal tubular epithelial cells and urothelial cells, respectively. Due to the different expression sites, SARS-CoV-2 cannot enter the renal parenchymal cells through the mechanism of action of ACE2 and TMPRSS2 coreceptors.46, 60, 61 Although the SARS-COV-2 virus particles observed under electron microscopy are considered controversial due to the reports from later studies,62 follow-up studies found that through reverse transcription-polymerase chain reaction,63-65 in situ hybridization,40, 66, 67 immune histochemistry,68, 69 immunofluorescence,55, 70 and other methods that SARS-CoV-2 protein and RNA are present in kidneys. Although the SARS-CoV-2 positivity rate of these detection methods is less than 50%,71 the possibility that SARS-CoV-2 directly infects the kidneys cannot be ignored. Another study found that CD147 may be involved in the process of SARS-CoV-2 virus invasion of the cells. CD147 is highly expressed in the damaged renal tubular epithelial cells and podocytes of COVID-19 patients.72-74 ALOP1 gene polymorphism and increased expression of CD147 in podocytes in SARS-CoV-2 patients may be involved in podocyte damage during COVID-19. SARS-CoV-2 infiltrates podocytes through CD147, leading to cytoskeletal structure and function disorders, stimulation of the silk mitogen-activated protein kinase pathway, and increased production of pro-inflammatory cytokines.75, 76
5.2 Cytokine storm
The occurrence of AKI after SARS-CoV-2 infection is mainly attributed to multiple organ failure, cytokine release syndrome, and exaggerated response of the body's immune system to SARS-CoV-2, resulting in an excessive inflammatory response called “cytokine storm.”4 Overproduction of proinflammatory cytokines causes damage to multiple organs by activating the macrophage activation syndrome receptor axis.77 The important proinflammatory cytokines IL-1, TNF-a, and IL-6 are mainly derived from macrophages, mast cells, endothelial cells, and epithelial cells; these cytokines participate in the body's natural immunity. After SARS-CoV-2 infection, the levels of cytokines increase sharply and they are released in large quantities into the blood, even at the site of infection. The cytokines act on endothelial cells and mucosal barriers, causing damage to the human tissues and organs. This damage leads to ARDS, AKI, and even multiple organ failure.8, 78, 79 A large number of studies have shown significantly increased proinflammatory factors in patients with severe SARS-CoV-2, including IL-1, IL-6, IL-7, IL-8, IL-9, IL-10, tumor necrosis factor alpha (TNF-a), interferon-gamma (INF-γ), monocyte chemoattractant protein-1 (MCP-1), and macrophage inflammatory protein-1 (MIP-1)4, 80; these proinflammatory factors play an important role in AKI in COVID-19 patients.
In some COVID-19 patients, mononuclear cell infiltration was found in the renal interstitium, possibly as a result of cytokine storm.81 The possible mechanisms include (1) INF-γ and TNF-α-mediated increases in the expression of signal transducer and activator of transcription-1 (STAT1) and interferon regulatory factor 3; (2) stimulation of macrophages by SARS- CoV-2 to secrete a large quantity of proinflammatory cytokines; and (3) allergic responses to anti-infective drugs.82-85
5.3 Abnormal activation of the complement system
Excessive activation of the complement system after SARS-CoV-2 infection results in immune-inflammatory response and target organ damage.86-88 Studies have reported significantly increased blood C5a level in severe COVID-19 patients.89, 90 C5b-9 and C4b are independent risk factors for respiratory failure in COVID-19 patients,91 and increased C3a level and C3a/C3 ratio are associated with an increased mortality in hospitalized patients.92 Kidney damage may also occur due to complement activation after SARS-CoV-2 infection, and patients with an elevated C3a/C3 ratio have a 1.9-fold higher incidence of severe AKI and treatment with renal replacement therapy than those with a normal C3a/C3 ratio.93 The deposition of complement components C5b-C9 form the membrane attack complex (MAC) in the tubular basement membrane, which may directly lead to the destruction of proximal tubular cells.94 At the same time, the cascade activation of the complement system may lead to chronic kidney inflammation, tubular atrophy, and interstitial fibrosis.95
5.4 Coagulation abnormalities
After SARS-CoV-2 infection, coagulation dysfunction may lead to various complications, such as venous thrombosis, ischemic stroke, myocardial infarction and liver and kidney damage.96 Studies have reported that abnormal coagulation function on admission (Activated Partial Thromboplastin Time, international normalized ratio, and fibrinogen) is a risk factor for AKI.97 A study reported that platelet-rich microthrombosis was found in the heart, brain, and lung tissues of COVID-19 patients.98, 99 Similarly, imaging and renal tissue examination showed that some COVID-19 patients experienced renal infarction and intrabulbar thrombosis.12, 100 These coagulation abnormalities may be related to the rapid increase in angiotensin II after SARS-CoV-2 infection, which induces cytokine storm, increases the secretion of von Willebrand factor (VWF)—A disintegrin and metalloproteinase with thrombospondin motifs 13 (ADAMTS13), and damages the vascular endothelial cells.101, 102 Excessive activation of the complement system to form the MACs may also lead to abnormal coagulation and thrombosis.103
6 CLINICAL MANIFESTATIONS OF COVID-19-ASSOCIATED KIDNEY INJURY
The main clinical manifestations of kidney injury after SARS-COV-2 infection are increased serum creatinine, blood urea nitrogen, AKI, proteinuria, hematuria, and renal infarction. The first study from China reported that among 701 patients, the incidences of elevated serum creatinine, blood urea nitrogen, and estimated glomerular filtration rate < 60 ml/min/1.73 m2 were 14.4%, 13.1%, and 13.1%, respectively. In addition, 43.9%, 26.7%, and 6.7% of patients had proteinuria, hematuria, and AKI during hospitalization according to the Kidney Disease Improving Global Outcomes (KDIGO), diagnostic criteria.1 A study from the United States reported that 36.6% of patients developed AKI after SARS-COV-2 infection. According to the KDIGO staging, the proportions of patients with stages 1–3 injury were 46.5%, 22.4%, and 31.1%, respectively. In 46.1% of the patients, the proportions of urinary occult blood and urinary protein (2–3+) were 46.1% and 42.1%, respectively. The proportions of urinary white blood cells and red blood cells were 36.5% and 40.9%, respectively. Furthermore, 65.6% of patients had urinary sodium < 35 mEq/L.9 The mortality rate of COVID-19 patients with CKD was 1.48–3.25-fold higher than that of patients without CKD.104, 105 A study from European renal association-European dialysis and transplant association reported that kidney transplant patients infected with SARS-COV-2 had a 1.28-fold higher risk of death than dialysis patients, with higher mortality rates among those aged > 75 years and with diabetes.26 A Spanish study found that the mortality rate of kidney transplant patients infected with SARS-COV-2 was related to the age of the patient and the time of kidney transplant: a shorter time since receiving the kidney transplant was associated with a higher mortality rate. The mortality rate was as high as 54.5% in patients aged ≥ 65 years who received kidney transplantation < 6 months previously.106 A meta-analysis of 5948 kidney transplant patients reported that after SARS-COV-2 infection in kidney transplant patients, 44% developed AKI, 12% required dialysis, 8% experienced transplant failure, and 21% died.107 In adolescents and children, nephrotic-range proteinuria and even nephrotic syndrome can occur after SARS-COV-2 infection, and patients with pre-existing nephrotic syndrome may experience disease relapse.108 Some patients developed hemolytic urine syndrome and antineutrophil cytoplasmic antibodies (ANCA)-related vasculitis.109-111
In addition, the mutated SARS-CoV-2 virus also has the risk of causing kidney injury. Gabriella reported a case of a Covid-19 Delta variant positive patient with diffuse thromboembolic disease, including right renal artery thrombosis.112 The COVID-19 vaccine has a certain protective effect on infection with SARS-CoV-2 mutant strains, and it was observed that four doses of mRNA vaccine can improve the neutralization effect of Delta mutant strains.113 A study in Mexico found that the mortality rate of kidney transplant patients infected with SARS-CoV-2 was 26.91% (130/486). After the virus mutates, because of the different types of mutations, the risk of mortality is different. The highest mortality rate was the b.1.1.220 variant strain, with a mortality rate of 30.43%, and the mortality rate of Omicron was 16.41%. The mortality before and after vaccination was 30.94% and 23.46%, respectively.114
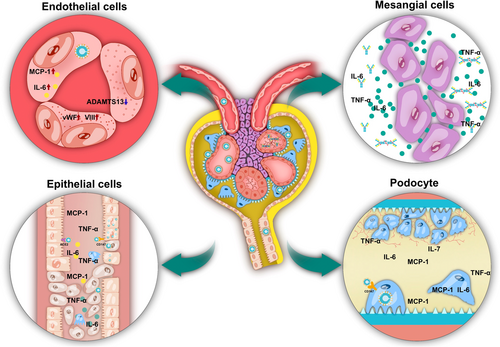
7 PATHOLOGICAL CHANGES RELATED TO COVID-19-RELATED KIDNEY INJURY
During the early stage of COVID-19 infection, studies were focused on the direct and indirect renal damage caused by SARS-COV-2 to the kidneys. In summary, the main basis of renal injury caused by SARS-COV-2 is the destruction of renal parenchymal cells, such as renal tubular epithelial cells, endothelial cells, podocytes and mesangial cells (Figure 2). The main mechanisms are as follows: (Table 2)
| Type of cell | Mechanism | Histological changes of kidney | Reference |
|---|---|---|---|
| Tubular epithelial cells | Possible direct infection with SARS-CoV-2; Abnormal immune responses caused by increased proinflammatory factors and chemokines; induction of hypoperfusion, drugs, hypertonic fluids, and rhabdomyolysis. |
Epithelial cell shedding, vacuolar degeneration, and necrotic debris casts in the tubular; The brush border of epithelial cells is sloughed off, and the cells are flat or new; tubulitis. |
52-55, 107 |
| Endothelial cells | Endothelial activation and endothelial dysfunction after SARS-CoV-2 infection; IL-6, IL-1, TNF-a and other proinflammatory cytokines increased; Monocyte chemoattractant protein-1; Von Willebrand factor activity, and factor VIII are elevated; ADAMTS13 decreased. |
Viral inclusion particles were seen in glomerular endothelial cells; Endotheliitis; Erythrocyte filling in the glomerular capillary; loops, thrombosis; COVID-19-associated vascular inflammation; COVID-19-associated coagulopathy. |
108-113 |
| Podocytes | SARS-CoV-2 enters podocytes through CD147, causing destruction of cytoskeleton structure and function, stimulating the MAPK pathway, and increasing proinflammatory cytokine production; APOL1G1 polymorphisms lead to mitochondrial dysfunction and impaired integrity of the glomerular podocyte membrane |
Diffuse fusion of podocyte foot processes, focal segmental glomerulosclerosis, podocyte shedding, and pseudocrescent formation. | 65, 114-117 |
| Mesangial cells | Cytokine storm induces B cells to produce IgA and IgG, form immune complexes; SARS-CoV-2 spike protein acts as a superantigen to stimulate T cell activation and the production of inflammatory mediators. |
Glomerular mesangial cells and mesangial matrix hyperplasia; Mesangiolysis with loss of mesangial cells; IgA, IgG, C3 deposition in the mesangial area and capillary loops. |
55, 118-120 |
- Abbreviations: IgA, Immunoglobulin A; IgG, Immunoglobulin G; MAPK, mitogen activated protein kinase; SARS-CoV-2, severe acute respiratory syndrome coronavirus-2.
7.1 Tubular epithelial cells
Mechanisms leading to renal tubular epithelial cell injury include: possible direct infection with SARS-CoV-2; abnormal immune responses caused by increased proinflammatory factors and chemokines; induction of hypoperfusion, drugs, hypertonic fluids, and rhabdomyolysis.56-59 This results in diffuse damage to renal tubular epithelial cells, including epithelial cell shedding, vacuolar degeneration, and necrotic debris casts in the tubular. The brush border of epithelial cells is sloughed off, and the cells are flat or new; tubulitis.12
7.1.1 Endothelial cells
After SARS-CoV-2 infection, vascular endothelial activation and endothelial dysfunction lead to the elevation of proinflammatory cytokines such as IL-6, IL-1, and TNF-a.117 MCP-1, vWF activity, and factor VIII levels are elevated, and ADAMTS13 is decreased, and these imbalances may lead to hypercoagulability, leading to thrombosis.118, 121 we can see viral inclusion particles in glomerular endothelial cells; endotheliitis119; Renal artery thrombosis120; erythrocyte engorgement in the glomerular capillary loops, and COVID-19-associated vasculitis and coagulopathy.55, 119
7.1.2 Podocytes cells
SARS-CoV-2 enters podocytes via CD147, causing disruption of cytoskeletal structure and function, stimulating the mitogen activated protein kinase pathway, and increasing proinflammatory cytokine production81; in addition, APOL1G1 polymorphisms leading to mitochondrial dysfunction and impaired integrity of glomerular podocyte membrane can cause podocyte destruction.40, 41 In kidney pathology, we can see diffuse fusion of podocyte foot processes, focal segmental glomerulosclerosis, podocyte shedding, and pseudocrescent formation.40, 122-124
7.1.3 Mesangial cells
Cytokine storm induces B cells to produce Immunoglobulin A (IgA) and IgG, form immune complexes, and deposit in glomeruli125; SARS-CoV-2 spike protein acts as a superantigen to stimulate T cell activation and the production of inflammatory mediators,126 mesangiolysis with loss of mesangial cells.127 Resulting in the proliferation of glomerular mesangial cells and mesangial matrix hyperplasia, IgA, IgG, C3 deposition in the mesangial area, and capillary loops.128
7.1.4 Kidney pathological diagnosis
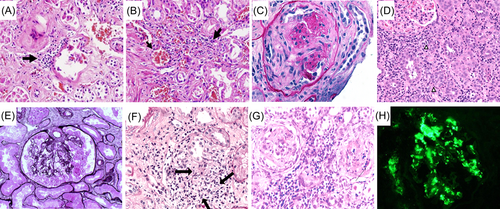
The renal pathological examination and autopsy findings showed that COVID-19 patients with kidney injury have obvious renal pathological changes, including in the glomeruli, renal tubules, and renal interstitium. However, tubulointerstitial injury is significantly more serious and manifests in renal tubules as detached brush border, exposed tubule basement membrane, and the presence of numerous granular casts in the lumen67, 129 (Figure 3A,B). With the increase in cases of kidney damage caused by SARS-COV-2 infection and the improved understanding of the pathogenesis, it was found that the kidney damage related to SARS-COV-2 infection also includes crescentic glomerulonephritis caused by ANCA-associated vasculitis110, 130, 136 (Figure 3C), thrombotic microangiopathy,137, 138 acute tubulointerstitial nephritis131, 139 (Figure 3D), and collapsing glomerulopathy. It is generally believed that collapsing glomerulopathy is related to ALOP1 gene mutations, and 36% of patients can achieve partially alleviation of the proteinuria76, 132, 140 (Figure 3E). Some patients may also develop granulomatous interstitial nephritis133 (Figure 3F), IgA nephropathy, and glomerulonephritis-crescentic immunoglobulin A nephropathy134, 135 (Figure 3G). There are also rare reports of membranous nephropathy and anti-glomerular basement disease associated with disease relapse in COVID-19.141, 142 With the increasing use of COVID-19 vaccination worldwide, many types of kidney injuries related to COVID-19 vaccination have also been reported. The main pathological types of injury include ANCA-associated vasculitis,143 IgA nephropathy,144 immunoglobulin A vasculitis,145 acute interstitial nephritis,146 and minimal change disease.147, 148 However, most data on kidney damage related to COVID-19 vaccination are presented in case reports. The incidence of minimal change disease is slightly higher in COVID-19 patients and is more common in the adolescent population. However, the incidence of minimal change disease is higher in the adolescent population in unvaccinated individuals.149 The striking similarity in kidney pathology between human immunodeficiency virus-associated nephropathy. The identification of the type of kidney injury in patients depends on renal pathological examination, except that some patients have positive antineutrophil cytoplasmic antibody. They were identified by light microscopy, immunology and electron microscopy.
8 TREATMENT OF COVID-19-ASSOCIATED KIDNEY INJURY
Since treatments for AKI do not differ according to etiology, the treatment principles for COVID-19-related AKI developed by the Acute Disease Quality Initiative are the same for other types of AKI,85 with differences in the management of hypovolemia and hemodynamic parameters. Hypovolemia is the main risk factor for indirect AKI caused by SARS-COV-2, so volume management is essential during the early stage. Dynamic monitoring of volume status and individualized volume management can reduce the occurrence of AKI. Limit fluid intake according to blood pressure and volume load. Other management principles of AKI vary according to the AKI grade. The management principles in AKI stage I include monitoring of the urine volume and serum creatinine, maintenance of blood sugars, avoidance of the use of nephrotoxic drugs and contrast agents, and selection of an appropriate assisted artificial ventilation mode. AKI stage II/III should be managed according to the status of renal function by avoiding the drugs that are metabolized or excreted by the kidneys, administering renal replacement therapy if necessary, avoiding the use of subclavian veins as the channel for hemodialysis, and nutritional management.85, 150 In addition, the use of remdesivir for viral infection in COVID-19 patients can reduce the occurrence of AKI and shorten the recovery time, and is safe.151-153 The use of glucocorticoids,154 immunoglobulins,155 and IL-6 receptor monoclonal antibody to treat the cytokine release syndrome can improve the survival rate of COVID-19 patients and reduce the need of renal replacement therapy.156-158 To a certain extent, the prognosis of patients can be improved, but high-dose immunoglobulin should be used carefully in patients with kidney disease.159 Traditional Chinese medicine has a certain effect in the treatment of COVID-19-related AKI by regulating core targets, such as Advanced Glycosylation End AGE-RAGE, PI3K-AKT, TNF pathway, and PTSG.160-162 Immunosuppressive therapy, including methylprednisolone, plasmapheresis, cyclophosphamide, mycophenolate mofetil, and rituximab, can cure patients with pre-existing renal disease, new-onset nephrotic syndrome, and concomitant hemolytic uremic syndrome, vasculitis, and acute necrotizing glomerulonephritis.108
8.1 Conclusions and recommendations
With continuous improvement in awareness related to COVID-19 and increased use of COVID-19 vaccination, COVID-19 is currently occurring intermittently and sporadically. However, there are still many problems associated with COVID-19,COVID-19 is closely related to kidney damage. Rapidly progressive kidney damage, such as AKI, vasculitic kidney injury, thrombotic microangiopathy, and hemolytic uremic syndrome, may occur in the early stage of COVID-19. Increasing types of pathological damage associated with COVID-19 may be identified in the future. At present, the treatment of COVID-19-related nephropathy is still based on the renal pathological type of patient, and the corresponding treatment is given. For example, patients with vasculitis are treated with glucocorticoids, cyclophosphamide, CD20 monoclonal antibody, and so forth. In the future, we can learn from SARS-Start with the mechanism of SARS-CoV-2 causing kidney disease and explore new treatment methods. Future studies should evaluate the disease spectrum, treatment, and prognosis of kidney damage after COVID-19.
AUTHOR CONTRIBUTIONS
Lirong Lin, Junhui Deng, Jie Li, Luquan Zheng, Zhifeng Wu, Wei Tan collected all the literature, carried out the analysis of data and outcome, Lirong Lin mainly drafted the manuscript. Jurong Yang revised and approved the final manuscript. Each author contributed important intellectual content during the drafting and revision of the manuscript. All the authors read and approved the final version of the manuscript to be published.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (No. 82270723, 81770682), The Chongqing Talent Program Project (cstc2021ycjh-bgzxm0090) and the Special project for technological innovation and application development of Chongqing (cstc2019jscx-msxmX0166).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in [repository name] at [DOI], reference number [reference number].




