Human monkeypox (hMPXV) re-emergence: Host immunity status and current vaccines landscape
Abstract
Monkeypox virus is a member of the Orthopoxvirus genus and the Poxviridae family. Orthopoxviruses are among the most intricate animal viruses. The pathogenicity of human monkeypox infection has been emphasized in response to its recent emergence in non-endemic countries and the threat of bioterrorism. It is always necessary to take appropriate precautions in exposure to emerging or re-emerging infections. Here, we focus on the current state of the human monkeypox infection outbreak, research & development of immune responses, and clinical interventions to prevent and treat the human monkeypox virus and other human poxviruses.
1 2022 HUMAN MONKEYPOX INFECTION OUTBREAK
On May 7, 2022, the first case of the human monkeypox virus (hMPXV) was confirmed in the United Kingdom, and the infectious disease alarm rang once more following the COVID-19 pandemic.1, 2 Monkeypox outbreak was declared a public health emergency of global concern by WHO on July 23, 2022. According to WHO, the Central African or Congo Basin clade will now be known as clade I, and the West African clade will be known as clade II. To avoid stigmatization, the WHO has been discussing renaming the virus. Recent monkeypox virus cases were more common in 18−44 years of age men who had sex with other men [people who self-identify as gay, bisexual, transgender, and nonbinary are included].3 It is unknown where the outbreak originated. What caused it to spread? Given the current outbreak's growing severity, it is critical that we focus on the rate of person-to-person transmission. When the genetic sequences of the virus obtained from infected patients are compared to previous sequences, we can determine whether the virus has mutated and whether the circulating virus will be more transmissible. Although monkeypox infection is not transmitted sexually, examination of sperm or vaginal samples may reveal new clues to the virus's spread. Changes in forest ecosystems allow opportunistic viral strains to spread, such as the Ebola or monkeypox viruses.4 According to the most recent CDC report, humans can spread diseases to dogs. It is unknown if infected animals can include cats, hamsters, gerbils, and guinea pigs.5
The global response to the monkeypox outbreak seeks to contain the disease's spread via the effective use of stringent public health measures. With the discontinuation of smallpox vaccination, the orthopoxviruses can spread to a significant portion of the current population. Several studies have found that those who received the smallpox vaccine had an almost 85% reduced risk of getting monkeypox than those who did not, as well as fewer severe symptoms and long-term complications from MPXV infection (39.5% vs. 74%) and lower mortality rates.6, 7 A study on the 528 cases identified during this outbreak found that just 9% of the infected individuals had ever received the smallpox vaccine.8 The United States, reported the first MPXV infection outside of Africa in 2003. Exposure to prairie dogs that had been infected by imported Gambian pouched rats led to this outbreak in humans. Israel, the United Kingdom, and Singapore were among the other countries with MPXV that were recorded.9
The MPXV outbreak in 2022, which affected both humans and cattle, as well as the rising number of immunocompromised people who cannot receive the vaccine have reignited interest in studying orthopoxvirus immunity and protective therapy. The latest findings provide a framework to study the humoral immune reaction given on by systemic orthopoxvirus infection.
2 GENETIC DIVERSITY AND PROTEINS DIFFERENCES IN POXVIRUSES
Determining whether the current monkeypox outbreaks are caused by genetic changes in the monkeypox virus is still a challenging scientific question to answer. The outbreak of lineage B.1 from West Africa in 2022 is its cause (MPXV Clade 3). At least 46 single-nucleotide polymorphisms that are unique to this lineage exist.10 For instance, the present outbreak may be caused by the MPXV strain that is circulating in the 2022 outbreak having more insertions and deletions in its DNA than the MPXV strains that were circulating before 2017.11 Surface glycoprotein B21R, a crucial antibody target, has three nonsynonymous single nucleotide polymorphisms (D209N, P722S, and M1741I) that may boost viral transmissibility.9, 11
In the form of genetic accordions [poxviruses can evade host antiviral responses by a series of gene activation and mutational processes], the vaccinia virus (VACV) is capable of developing mutations. The K3L gene has been amplified significantly in one strain of VACV, and this strain, along with a helpful point mutation in the same gene, allows the virus to effectively resist host PKR response. This mechanism for the evolution and spread of MPXV has not yet been proven.12
Currently, the known poxvirus host range genes are divided into 12 different classes, some of which have just one gene (e.g., K3L, E3L, K1L, etc.), and others of which have multiple members (e.g., serpins, C7L family, TNFRII family, etc.), most likely as a result of lineage duplication occurrences. These genes, like VACV and MPXV, which are linked to outbreaks of infections in humans and cattle, have historically been referred to as host range genes. It's possible for rodents to serve as reservoir hosts for these viruses. While other poxviruses are emerging zoonotic diseases that affect various animal species, including cattle, rodents, monkeys, and others, for example, variola virus (VARV), which have humans as their sole host. Skin lesions are the primary clinical manifestation of poxvirus infection.13
In poxviruses, host range and genetic diversity are significant. Apoptosis, interferon (IFN), and inflammatory pathways are just a few of the host antiviral and/or anti-inflammatory mechanisms that interact with the function of various host range genes.14 E3L and K3L VACV genes have received the most attention among the known host range genes reported in poxviruses. Protein kinase R (PKR) and 2′-5′ oligoadenylate synthase are both inhibited by the E3L-encoded protein because it contains a C-terminal double-stranded RNA-binding domain that probably prevents their activation by dsRNA and induction by IFN.15-17 Since it is homologous to the S1 domain of the subunit of eukaryotic translation initiation factor 2 (eIF2), the K3L protein acts similarly to the E3L protein by inhibiting the PKR activity.18, 19 It does this by acting as a pseudo-substrate and a competitive inhibitor of the kinase protein. SPI-1 is another well-known host range gene, however other serpins, such as Orthopoxvirus SPI-2/CrmA and SPI-3, three serpins in the Leporipoxvirus genus, and five serpins in the Avipoxvirus genus, may also contribute to poxvirus host range genes. By acting on many targets of coagulation, complement activation, and inflammation, the prominent in the poxvirus suppress the host's response to viral infection.20 Some poxviruses also have genes that encode proteins with short complement-like repeats called the B5R/VCP family. These proteins interact with an unidentified cellular surface molecule to activate Src kinase, which then causes actin to polymerize and promotes the spread of the virus from cell to cell.21, 22
Ankyrin repeat domains (ANK) are found in many of the proteins encoded by poxviruses, and the carboxy-terminal region of most proteins contains an F-box domain. The protein is called CP77 or CHOhr and has been recognized as a host range factor since the deletion of the poxvirus ANK protein in VACV resulted in a restriction of replication in a Chinese hamster ovary cell line.23
By interacting with the ANK repeats in the p65 subunit of nuclear factor KB (NF-B) and the F-box of the cellular SCF ligase complex, CP77 inhibits the activation of NF-B.24 Other ANK proteins might play similar roles, but additional studies must confirm this. E3L, C7L, and m62r (C7L family) are the genes that have homologs with the largest diversity of poxviruses, according to Oliveira et al.,13 the genus orthopoxvirus is the only one to contain F1L and an Ank-box homolog. Different genes crucial for their host range but have not yet been found are likely present in distantly related poxviruses.25
3 PATHOGENESIS AND KEY PROTEINS OF hMPXV AND OTHER HUMAN POXVIRUSES
The route of entry the virus uses to develop the initial infection has a significant influence on the clinical consequence of orthopoxvirus infection in a host.26 By infecting local immune cells in surrounding tissue, including perhaps antigen-presenting cells like monocytes, macrophages, B cells, and dendritic cells, viruses spread. There is controversy about the exact processes by which orthopoxviruses spread from the main site of infection to adjacent draining lymph nodes. For instance, VACV uses lymphatic veins for a diffusion mechanism.27, 28
Inflammation of the lymph nodes, often known as lymphadenopathy, is one of the symptoms of monkeypox. Following lymphatic tissue spread, MPXV may attack additional important organs like the spleen and liver. Notably, MPXV antigens have been found in Kupffer cells and hepatocytes in nonhuman primate (NHP) models. The virus may then spread to the farthest organs like the skin and gonads during the viremic cycle [respiratory droplets, body fluids, contaminated objects, skin lesions—the crust of an infected individual]. It is unknown if sexual contact directly promotes the spread of monkeypox, despite the fact that intimate physical contact is a known risk factor for transmission.29, 30 There is evidence that lymphoid organs including the spleen and bone marrow facilitate VARV replication, although liver involvement is less clear.
To develop newer therapeutic approaches or vaccines, it is necessary to understand the behavior of the significant proteins in the recent monkeypox virus outbreak, as well as how similar it is to previous outbreaks. The genus orthopoxvirus contains significant members such as variola, vaccina, and monkeypox, among others. The VARV which causes smallpox has been eradicated. Monkeypox virus is a common zoonosis threat that is considered a threat due to the global population's reduced immunological burden. After smallpox eradication, human monkeypox infection is now the most common orthopoxvirus infection. NHP such as cynomolgus and rhesus monkeys have been used to study orthopoxvirus infections. Nonetheless, the pathogenicity of human monkeypox strains from both African clades has been evaluated in cynomolgus monkeys.31 Human monkeypox pathogenesis, which causes systemic or fulminant infections, is still unknown. Neutrophils play an essential role in the pathogenesis of sepsis-related human monkeypox infections.32
Poxviruses have a double-stranded (ds) DNA genome, approximately 200 kb lengthy, encoding more than 200 genes.33 Poxviruses have distinct viral cycles. They enter the cell via pH-dependent endocytosis and macropinocytosis. Intermediate and late viral RNA transcription occur following the replication of the linear DNA genome in the cytoplasm. Noninfectious forms are then assembled, and further processing produces the first intracellular infectious form of the virus called intracellular mature virion (IMV). Sometimes, viral particles acquire two additional membranes from the trans-Golgi or endosomal membrane, called intracellular enveloped virion (IEV) or wrapped virion. Finally, due to the fusion of mature infectious virions with the cell membrane and viral shedding, the IEV is either released as an extracellular enveloped virion (EEV) or a cell-associated enveloped virion (CEV).34 Most poxviruses are in CEV form, but there are some exceptions. For example, the IHD-J strain of the VACV is primarily released in EEV form.35 Poxviruses' ability to infect a wide range of cells is justified by the recruitment of a large number of proteins. Poxviruses infect in two ways: the mature virion (MV), which has a single membrane, and the EEV, which has an additional external membrane.36 MV and EV are antigenically different. Meanwhile, MV is more stable and is considered a mediator of transmission between hosts. While EV escapes the host's immunity and is responsible for the intra-tissue spread of the virus37 (Figure 1). At least nine proteins are known to be involved in EV formation, including A33, A34, A36, B5, and others. A27, A26, D8, A17, A21, A16, L5, H2, and other proteins are involved in MV formation (entry fusion complex). Fusion is accomplished by 12 complex non-glycosylated membrane proteins. For cytoplasmic viral replication, poxviruses rely on virus-encoding proteins. The vaccina virus is responsible for our understanding of the pathogenesis of poxvirus proteins. According to the most recent research, there are 118 early genes, 52 intermediate genes, and 38 late genes.36
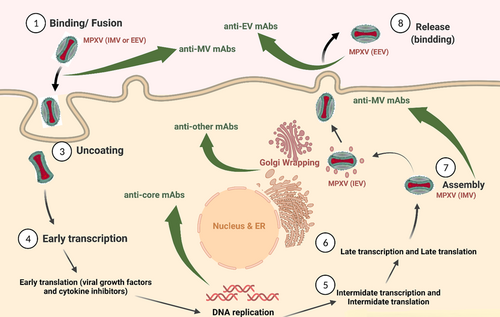
Albarnaz et al.38 discovered that the vaccina virus's F14 protein is a selective inhibitor of Nf-KB-dependent gene expression and increases virulence. It is encoded by other poxviruses, including the monkeypox virus. The genetic and amino acid differences between the two clades of the monkeypox virus may indicate differences in pathogenicity, but how this works is unknown. Because smallpox vaccination is limited in some parts of the world, the monkeypox virus has become a more potent human pathogen.
The COP-B7R and BR-203 ortholog genes are virulence factors in the monkeypox virus but not in the VARV. COP-H5R (viral transcription factor), COP-A9L (morphogenesis factor), COP-A50R (DNA ligase), and COP-A36R (actin tail formation) are also viral life cycle genes that affect monkeypox virus replication and transcription, in addition to the virulence gene ortholog family. The monkeypox virus contains genes encoding proteins such as COP-B19R (IFN alpha/beta binding factor), BR-207 (apoptosis factor), and BR-05-226 (TNF binding factor), but they are fragmented in the vaccina virus.
4 IMMUNITY TO ORTHOPOXVIRUSES
Various immunological processes underlie cross-protection conferred by vaccination against the VACV, and most of them involve neutralizing antibodies (NAbs). The passive transfer of sera from VARV or VACV-immune individuals protects exposed individuals against smallpox, which highlights the critical role that antibodies (Abs) play in orthopoxvirus protection. Recent research in nonhuman experimental ape or mouse infection models has demonstrated that polyclonal Abs are both essential and sufficient for protection against lethal challenges with MPXV or VACV. The best indicator of how effectively humans will be able to combat orthopoxvirus infections in vitro is the amount of neutralizing activity present in the immune serum.39, 40 Because of the wide variability in potency and an incomplete understanding of the molecular mechanisms regulating protection, the degree of efficacy is unknown. A vast spectrum of serum Ab responses targeting numerous VACV antigenic determinants are produced after percutaneous inoculation with VACV. Sera from immune persons that have cross-neutralizing activity against VACV, MPXV, and VARV are likely made up of Abs with various specificities.41 The surface proteins of both the EV and MV VACV virion types are among the several antigenic targets that vaccinia immune globulin (VIG) may detect. Polyclonal antibody research in rabbit immunological serum revealed that whereas each orthopoxvirus had a distinct pattern of recognition, they all shared neutralizing components. Studies in mouse infection models identified MV surface proteins A27, L1, H3, D8, A28, A13, and A17 as well as EV surface proteins B5 and A33 as targets for neutralizing and protective murine monoclonal antibodies (mAbs). Furthermore, no one is aware of the essential characteristics of mAbs required and sufficient for the human B-cell response to provide protection against orthopoxviruses.42
When mice lacking in B cells were later challenged intranasally with the VACV Western Reserve strain (VACV WR), it was discovered that the Dryvax vaccination had provided protection, demonstrating agreement in the immune responses. Similar to this, mice that had received skin scarification vaccination with either a recombinant VACV or a modified Ankara vaccinia virus (MVA) were protected from a respiratory challenge as long as either antibody or T cells were present.43, 44 Strong humoral reactions brought on by the smallpox vaccine are essential for disease prevention.45 Neutralizing serum antibody titers >1:32 were linked to protective immunity against smallpox disease.46 Orthopoxviruses continue to pose a threat to the worldwide health of infectious diseases. The capacity of VACV smallpox vaccines to stimulate cross-protective Abs against other OPVs and serve as the foundation for recombinant vaccines against illnesses like rabies are significant characteristics of these vaccines. Despite being used to eradicate smallpox, first-generation vaccines had a long list of adverse effects, including eczema, progressive vaccinia, and myopericarditis.47 Acam2000 and Imvamune, two second- and third-generation vaccines, were created in response to these concerns.48, 49 The extent and quality of the response, the safety of immunocompromised people, and the best utilization strategies are still up for debate.
5 MONKEYPOX INFECTION IMMUNITY
In studies, interactions between different orthopoxviruses and VACV are typically used to interpret immunity against monkeypox. According to studies,50-52 poxviruses mostly target monocytes. Poxvirus antigen in neutrophils and monocytes has been proposed as a critical determinant of MPXV fatality. Furthermore, it was discovered that primary human M2-like macrophages permit VACV replication and spread. These primary macrophages developed actin tails, cell junctions, lamellipodia, and branching structures linked to VACV virions after becoming infected, indicating that these cells might help the virus spread. However, it has also been found that innate immune cell depletion did not stop the spread of VACV in infected mice, indicating that other immune cells besides monocytes and macrophages are also able to disseminate the virus.53 Like monocytes, natural killer cells are an important part of the body's innate immune system and can change how the adaptive immune response works. When rhesus macaques get infected with MPXV, the number of natural killer cells in their blood and lymph nodes goes up by a lot.54 Animal models of VARV infection cause cytokine responses that are linked to how sick the animal gets. It has been demonstrated that human IFN prevents MPXV replication and dissemination. However, MPXV did not significantly activate TNF-and NF-B-regulated genes, especially in infected animals. This is not unexpected considering that TNF55 and NF-kB56 pathway modulator genes are present in VARV and other orthopoxviruses. Several cytokines, like IL-1, IL-1RA, IL-2R, IL-4, IL-5, IL-6, and IL-8, are higher in humans who have been infected with MPXV, and this is true no matter how bad the disease is. By suppressing inflammatory and antiviral immune responses (CCL2, IL-2, IL-13, IL-15, IL-17, and CCL589), VACV can evade immunological responses. MPXV may employ a similar tactic to trick host immunity. Numerous innate immune cells, such as monocytes or macrophages, neutrophils, natural killer cells, conventional dendritic cells, plasmacytoid dendritic cells, and innate lymphoid cells, are yet unidentified in MPXV-infected patients. Understanding these immune cells' roles and locating critical indicators for disease prognosis requires characterization and profiling during MPXV infection. See reference29 for more details on orthopoxvirus immune evasion.
6 ANTIBODY IN RECOVERY POXVIRUS INFECTION
The first evidence of the importance of B cells and immunoglobulins against poxviruses was provided by the successful global immunization campaign that eradicated smallpox, which used a live VACV vaccine.57, 58 Further research established the effectiveness of VIG therapy in preventing infection in close relatives of smallpox patients. It is expected that cross-protective immunity to monkeypox may decline with time as well. Human vaccine cross-reactive VACV-induced immunoglobulins have recognized MPXV proteins. The MPXV proteins, D8, H3, and A26, were mainly targeted by NAbs against MPXV-infected macaques.59
NAbs, whether from a previous infection or a vaccine, play an important role in controlling viral infections. The majority of mAbs are based on IgG recognition. These mAbs are detected by the complement system or the Fc receptor, which is found in the majority of immune system cells. As a result, mAb-mediated immunotherapy can influence viral propagation via a variety of mechanisms. Functional Abs differ for each viral antigen as well. According to Pelegrin et al.,60 vaccine-like mAbs can stimulate both cellular and humoral immune responses. Due to safety concerns, the vaccina virus-based smallpox vaccine is not considered a prophylactic vaccine to prevent orthopoxvirus infection. Therefore, other treatments should be regarded for patients with more severe human orthopoxvirus infections. As first-line therapy, postexposure smallpox vaccination or immunoglobulins are viable options.61 Xiao et al.,62 stated that direct smallpox vaccination after exposure can protect people from serious illnesses and that revaccination in the first week after exposure can prevent smallpox.
The only product approved by the FDA to treat the side effects of VACV vaccination is VIG, which is effective in systemic orthopoxvirus infections caused by smallpox or monkeypox viruses. The new VIG stock is administered intravenously, which has better pharmacokinetic effects than the previous case, which was administered intramuscularly. VIG activity is evaluated against EV due to the importance of EV (EEV) in pathogenesis and the more stable MV. The ability of the VIG to neutralize MV defines its potency MV (IMV). The primary goal of VIG is to elicit EV-NAbs against the EV-specific B5 protein.63
Due to the common serological cross-reaction between orthopoxviruses, VIG can react with proteins of other orthopoxviruses (such as MPXV) and have similar therapeutic effects against other poxvirus infections. According to Kempe's study,64 the person who recommended VIG for the clinical treatment of smallpox vaccination complications, one of the complications after smallpox vaccination is a delay in the production of vaccine-induced Abs (Eczema vaccinatum). Therefore, both cellular and humoral immune responses are required to control viral infection. Although no clinical trial has been performed to evaluate the efficacy of VIG, it has become an accepted clinical treatment.
The VIG has been studied in animal models to treat poxvirus infections following exposure. Law et al.,65 found that anti-vaccinia rabbit Abs on the third day after infection had the same effect as Abs 1 day before the infection. Similarly, Lustig et al.,66 achieved the same results with VIG against smallpox vaccination. A single dose of VIG administered 1 day before and 1 day after the challenge provides complete infection protection. Thus, the difference in disease progression affects morbidity.
In general, it is claimed that while VIG is effective in the early stages of treatment for eczema vaccinatum, it is ineffective in the treatment of progressive VACV and smallpox virus. Furthermore, problems with VIG use, such as low doses and limited potency, have led to the development of alternative anti-poxviruses immunotherapy, as the side effects of conventional-related vaccines may still occur. These pitfalls have led to a reversal of therapeutic pathways to improve biodefense ability by enhancing the function of VIG, which includes mAbs to increase VIG potency or creating a cocktail of monoclonal or polyclonal Abs.
Because of the various infectious forms associated with poxviruses, selecting an antigen for antibody therapy is complicated. Human donors are required for the development of a specific set of mAbs against poxviruses. To find a suitable alternative to VIG, mAb development against the enveloping proteins of poxviruses in both EV and MV forms is a good option.67 Neutralizing antibody targets on MV and EV envelope proteins are encoded by A27L, A28L, D8L, H3L, L1R, and A33R, B5R genes, respectively. A protein encoded by the A27L gene is one of the potential targets for neutralizing mAbs. Among orthopoxviruses, this protein is highly conserved. More robust mAbs therapy results support cases in which the antibody combination binds to at least one target on both the MV and the EV to improve its therapeutic effect (Table 1).
| Positive response | |||
|---|---|---|---|
| Activity of neutralization | Protein | Human | Nonhuman primates |
| MV-neutralizing mAbs | WR | +++ | +++ |
| A13 | +++ | +++ | |
| H3 | +++ | +++ | |
| D8 | +++ | +++ | |
| A17 | +++ | ++ | |
| A27 | ++ | + | |
| A9 | + | + | |
| L1 | ++ | ++ | |
| MV-neutralizing mAbs | A56 | + | +++ |
| B5 | + | ++ | |
| A33 | + | + | |
| A36 | + | + | |
| A34 | + | + | |
| Core-neutralizing mAbs | WR70 | +++ | +++ |
| A10 | ++ | + | |
| L4 | + | + | |
| Evasion | E3 | + | + |
| Virulence factor | A27 | ++ | +++ |
| L1 | ++ | ++ | |
| H3 | ++ | ++ | |
| D8 | ++ | ++ | |
| Other | D13 | +++ | +++ |
- Note: Anti-EV or anti-MV monoclonal antibodies alone protect against severe poxviruses, but their combination provides more complete protection against more severe diseases. These data support the replacement of human mAbs for VIG.
- Abbreviations: EV, enveloped virion; mAbs, monoclonal antibodies; MV, mature virion; VACV WR, vaccina virus strain Western Reserve; VIG, vaccinia immune globulin; +, low antibody response; ++, moderate antibody response; +++, high antibody response.
Primary viral inoculation is controlled by anti-MV Abs, while the established viral infection is prevented by anti-EV Abs. Anti-EV antibody mechanisms are more complex than anti-MV antibody mechanisms in neutralizing poxviruses. Plaque reduction neutralization test and spread inhibition assay or comet reduction assay is used to neutralize MV and EV, respectively.62 Two mAbs of chimpanzee/human anti-B5 that had a high affinity to B5 could prevent the formation of comets in variola and vaccina viruses in vitro.72 Three Abs of chimpanzee/human anti-A33 with a high affinity to A33 could prevent comet formation in the VACV in vitro.70 Anti-poxvirus Abs not only neutralize them but also increase the Fc domain function of Abs by using effectors. For example, in the presence of a complement, anti-B5 mAbs with a suitable Fc domain have synergistic activity. Human anti-B5 mAbs with a positive effect on an antibody's Fc domain can reduce complement-dependent neutralizing activity and complement-dependent cytotoxicity in vaccina-infected cells.73
In addition to the therapeutic Abs described in detail above that target virus-enveloped proteins, other therapeutic Abs target proteins involved in poxvirus virulence. For instance, poxviruses (such as the vaccinia or VARVs) encode several immune response modifiers. One of these is type I IFN binding protein, which is appropriate for antibody targets. Anti-L1 and anti-A33 mAbs neutralize L1 protein and do not spread VACV EV in vitro. These combination Abs, particularly anti-L1 with A33 and anti-B5 with L1, will provide greater postchallenge protection.69
Finally, previous studies have shown that Abs against live orthopoxviruses provide better protection. The lack of complete protection has been attributed to a lack of EV-specific Abs. Abs play the most critical role in vaccine-mediated protection against orthopoxviruses. Passive administration of neutralizing mAbs is necessary due to the unpredictable nature of emerging and bioterrorism-related infections, such as the recent case of the monkeypox virus.
7 EFFECTIVENESS OF THE MONKEYPOX VACCINE
It is general practice that vaccinations for smallpox also provide cross-protection against monkeypox. Two factors are the primary causes of this cross-reactivity. So, first of all, there is a considerable measure of sequence similarity between orthopoxviruses, especially in immunologically significant proteins, which results in many immune epitopes that are shared. The broad scope of the response, with Abs, concentrating on at least 24 membrane and structural proteins. Similar to this, T-cell responses identify epitopes among a wide variety of viral proteins, with CD4+ T cells favorably detecting structural proteins and CD8+ T cells focusing on proteins produced early (e.g., virulence factors) in the viral life cycle.74, 75
NAbs have been proven to correlate with protection against variola, the virus that causes smallpox in humans, and other orthopoxviruses in animal models. T cells help to eliminate viruses even though they are not necessary for protection.
The absence of efficacy data from human clinical trials was confirmed by WHO on August 17, 2022. Based on human immune responses to Jynneos and information from an MPXV challenge research carried out in NHP, the effectiveness of a two-dose series of Jynneos against monkeypox was evaluated. Accordingly, the CDC76 and FDA stated that there is proof Jynneos can treat disease brought on by this strain of the monkeypox virus.77
The cross-immune response between poxviruses has been indicated by nonclinical results from various animal models, including primates, that show similar cross-humoral responses and protection against lethal challenges with monkeypox virus and vaccinia compared with prior generation smallpox vaccines.78
Even during the highest immune response, two doses of Jynneos did not prevent monkeypox infection in animal trials. Furthermore, the type of MPXV, such as the Indian strain A.2 that belongs to the HMPXV-1A lineage of clade 3 (West African clade), or the US and European types B1 may affect the efficiency of the monkeypox vaccination.79, 80 There are currently no data on the administration of the Jynneos vaccine together with other vaccinations. When recommending Jynneos and the COVID-19 vaccination, there are other factors to consider. Due to the observed risk for myocarditis and/or pericarditis after receiving ACAM2000 and mRNA vaccines, as well as the unknown heart health following the Jynneos vaccination, it is advised that young adult males wait 4 weeks before receiving Moderna, Novavax, or Pfizer-BioNTech vaccine.
The efficiency of the ACAM2000 smallpox vaccine in producing VACV-specific T cell responses was demonstrated by a study that revealed the presence of active CD4+ and CD8+ T cell responses at 1, 3, 6, and 12 months after immunization. Activated CD8+ T cells produced much larger amounts of CD107, a marker for degranulation, as well as IFN, TNF, IL-2, and CCL4 in response to VACV stimulation, indicating the presence of powerful memory T cell responses.81 VACV-specific CD8+ T lymphocytes produced IFN, TNF, IL-2, and CCL4 after activation. Both ACAM2000 and Dryvax led to the production of VACV-NAbs in the recipients of the vaccines, despite apparent variations in the targeted viral proteins.81-83
Due to the lack of clinical efficacy data, adolescents with monkeypox infection are eligible for postexposure prophylaxis with vaccination, immune globulin, or antiviral drugs. Fourteen days following the second dose of Jynneos, optimal immunity is achieved. It is unknown how long immunity seems to last.84 After receiving just one dose of IMVANEX,® breakthrough monkeypox infections were observed by Thy et al.85 Twelve of the 276 vaccine recipients experienced a breakthrough disease without developing a serious infection. Before receiving their full vaccine, 10 out of 12 patients contracted MPX infection within 5 days following vaccination. Days 22 and 25 saw the infection of two patients. The idea of reinfection should highlight how crucial it is to administer a second dose of vaccine on Day 28 (Table 2).
| No | Vaccine | Platform | Delivery route | Status |
|---|---|---|---|---|
| 1 | IMVANEX | A live, nonreplicating vaccine developed from modified vaccinia Ankara (MVA) | Two doses, s.c. | Protection against the vaccinia virus and the monkeypox in adults. |
| 2 | Jynneos | A live, nonreplicating vaccine developed from modified vaccinia Ankara/attenuated (MVA) | Two doses, s.c. | Vaccination against the smallpox and the monkeypox in people 18 years of age and elder. |
| The two doses should be separated by 28 days. | ||||
| 3 | Aventis | Vaccinia virus with live replication (NY strain) | One dose | Used in an emergency with smallpox. |
| Pasteur | ||||
| Smallpox | ||||
| Vaccine | ||||
| 4 | IMVANEX | A live, nonreplicating vaccine developed from modified vaccinia Ankara/attenuated (MVA) | Two doses, s.c. | Protection against the vaccinia virus and the monkeypox in adults. |
| 5 | Imvamune | A live, nonreplicating vaccine developed from modified vaccinia Ankara/attenuated (MVA) | Two doses, s.c. | Adults who are actively immunized against smallpox, monkeypox, and diseases & infections caused by a related orthopoxvirus. |
| 6 | Smallpox vaccine | vaccinia virus with live replication (NYCBH strain) | One dose | Used in an emergency with smallpox. |
| 7 | LC16m8 | Live, replicating attenuated vaccine (Lister strain) | Single dose | Vaccination against smallpox. |
| 8 | TNX-8011 (Tonix Pharmaceuticals Holding Corp.) | A preclinical live virus vaccine candidate developed on horsepox | Phase 1 study in Africa in 2023 | Protect against smallpox and monkeypox in people. |
| 9 | EpiVax (epitope-driven vaccine) | Preclinical phases | - | To predict a candidate for the smallpox vaccine. |
| 10 | VennVax | A multi-T-cell epitope poxvirus vaccination with DNA priming and peptide boost | - | Preliminary research on potential monkeypox vaccinations. |
| 11 | Moderna_tx | Preclinical phases | - | Moderna is conducting preliminary research on potential monkeypox vaccinations. |
| 12 | Blue water vaccines | Using the norovirus envelope and protrusion virus-like particle platform as a new monkeypox vaccine | - | Preliminary research on potential monkeypox vaccinations. |
- Abbreviation: S.C, subcutaneous injections.
8 CONCLUSION AND PROSPECTIVE
Human monkeypox infection is an emerging viral pathogen. Serological analyses in African clades show that the virus causes more infections than previously thought. When a high-risk strain of the monkeypox virus is introduced in opportunistic situations where the population is not immune to poxviruses, the virus is able to control this naive population and cause an epidemic. The difference in virulence severity among orthopoxviruses may be described by differences in proteins that affect the host's immune system. For example, the ortholog of the COP-C3L gene (a complementary control protein) between central and West African clades of the monkeypox virus may play a role in the virulence between the more infectious or less infectious strains of the monkeypox virus. Understanding this may aid in determining what causes the monkeypox virus to become a successful human pathogen and developing more effective treatments.
AUTHOR CONTRIBUTIONS
Conceptualization: Milad Zandi. Writing—original draft preparation: Maryam Shafaati. Writing—review and editing: Milad Zandi and Maryam Shafaati. Revised editing: Maryam Shafaati. Supervision: Milad Zandi. All authors have read and agreed to the published version of the manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




