Characterization and application of a series of monoclonal antibodies against SARS-CoV-2 nucleocapsid protein
Liling Zhou, Chuncong Mo, and Yujie Yang contributed equally to this study.
Abstract
The ongoing coronavirus disease 2019 (COVID-19) pandemic has a significant global social and economic impact, and the emergence of new and more destructive mutant strains highlights the need for accurate virus detection. Here, 90 monoclonal antibodies (MAbs) that exclusively reacted with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) nucleocapsid protein (NP) were generated. These MAbs did not cross-react with NPs of common human coronaviruses (HCoVs, i.e., 229E, OC43, HKU1, and NL63) and Middle East Respiratory Syndrome Coronavirus. Subsequently, overlapped peptides in individual fragments (N1–N4) of NP were synthesized. N1-3 (25-GSNQNGERSGARSKQ-39), N3-1 (217-AALALLLLDRLNQL-230), and N4-8 (393-TLLPAADLDDFSKQL-407) were identified as major epitopes using enzyme-linked immunoassay (ELISA) and recognized by 47, 1, and 18 MAbs, respectively. The 24 remaining MAbs exhibited no reactivity with all synthetic peptides. Among MAb-epitope pairs, only MAbs targeting epitope N1-3 displayed no cross-reaction with NPs of SARS-CoV-1 and other SARS-related CoVs. All Omicron variants contained a three-amino acid deletion (31ERS33) in the N1-3 region. Thus, MAbs targeting N1-3 failed to recognize these variants. Furthermore, a double-antibody sandwich ELISA for antigen detection was established using the optimal MAbs. Overall, a series of MAbs targeting SARS-CoV-2 NP was prepared, characterized with epitope mapping, and applied for the detection of SARS-CoV-2 antigens, and some novel B-cell epitopes of the viral NP were identified.
1 INTRODUCTION
The ongoing coronavirus disease 2019 (COVID-19) pandemic was caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a member of the species severe acute respiratory syndrome-related coronavirus (SARSr-CoV) of the genus Betacoronavirus in the family Coronaviridae; the virus has since mutated resulting in the emergence of different variants. The pandemic has further led to unprecedented global social and economic disruption. Therefore, diagnostic tests should be urgently developed.1-3
There are seven coronaviruses (CoVs), which are transmissible between human beings: SARS-CoV-2, SARS-CoV-1, Middle East Respiratory Syndrome Coronavirus (MERS-CoV), and four common human CoVs (HCoVs), namely, HCoV-229E, HCoV-OC43, HCoV-NL63, and HCoV-HKU1.4 SARS-CoV-2, SARS-CoV-1, and some bat CoVs, such as RaTG13 strain, are assigned to SARSr-CoV.5 Notably, the nucleocapsid protein (NP) of coronavirus shows the smallest variation among structural proteins. NP has several critical functions, such as binding to RNA to form viral ribonucleoprotein, coordinating intercellular and virion assembly phosphorylation, participating in viral RNA transcription and replication, and regulating host cell gene expression in virus-infected cells.6-8 NP also induces humoral and cellular immune responses after infection.4, 9, 10 Therefore, NP is a target for diagnosis and drug design.
In the early stages of COVID-19 infection, patients show more sensitive antibody responses to the NP than to the spike protein.11 Hence, serum antibody detection has been developed as one of the key diagnostic methods for patients with COVID-19. For instance, serological tests have been developed through a click-based strategy for microarrays with tailored peptides that have a strong immune response to the SARS-CoV-2 NP region leading to virus immobilization. Therefore, a better understanding of the structure of the SARS-CoV-2 NP, as well as its structural differences from related coronavirus NPs, may contribute to the development of sensitive and specific immunological tests.
Several methods have been developed for COVID-19 diagnosis, including molecular diagnosis via reverse transcription-polymerase chain reaction (RT-PCR) and serological tests detecting antibodies against NP.1, 12, 13 However, rapid, low-cost, and reliable SARS-CoV-2 antigen assays are still necessary for early screening of potential SARS-CoV-2 infected cases.14 SARS-CoV-2-specific monoclonal antibodies (MAbs) that target NP, the predominant viral protein detectable in clinical samples, are extensively applied to the development of such antigen testing methods.15
The present study explored SARS-CoV-2 NP epitopes using 90 MAbs. The cross-reaction of the MAbs with NPs of six other HCoVs was further investigated, and an antigen-capture sandwich enzyme-linked immunosorbent assay (ELISA) was developed based on the performance of these MAbs.
2 METHODS
2.1 Development of MAbs
The recombinant NP of SARS-CoV-2 was expressed in Escherichia coli for subsequent mouse immunization, and the mouse spleen cells were extracted. A series of antibody screening, subcloning, and further antibody determination experiments was conducted in accordance with the experimental operating procedures of the hybridoma MAb preparation technology. Subsequently, the mice were immunized via injection of the selected hybridoma cells, and their ascites were collected. A rabbit MAb (RAb) expressed in human embryonic kidney 293 cells was stored and prepared by Sino Biological Inc. RAb was used to detect the linear epitope N4-8 (393TLLPAADLDDFSKQL407) in NP of SARS-CoV-2.16
2.2 Recombinant proteins and peptides
Recombinant NPs of MERS-CoV, HCoV-229E, HCoV-OC43, HCoV-NL63, and HCoV-HKU1 expressed in E. coli, and NP of SARS-CoV-2 expressed in baculovirus-insect cells were obtained from Asbio Technology Inc. Additionally, recombinant NPs of SARS-CoV-1 and SARS-CoV-2 omicron BA.4 expressed in baculovirus-insect cells were obtained from Sino Biological. Four truncated recombinant SARS-CoV-2 NP fragments (N1: 1-120aa, N2: 106-225aa, N3: 211-330aa, and N4: 316-419aa) with N-terminal glutathione of S-transferase tag were expressed in E. coli, wherein 15 amino acids were found to overlap in the proteins.16
Thirty-six short peptides of approximately 15 amino acids in length, overlapping the full-length SARS-CoV-2 NP, were synthesized by GL Biochem Co. Ltd. (Supporting Information: Table S1). The peptides were purified (90%) using high-performance liquid chromatography and identified using mass spectrometry analysis. Some peptides were coupled with keyhole limpet hemocyanin (KLH) to increase the robustness of epitope encapsulation. One peptide from adenovirus 100k protein was synthesized as the control blank peptide to ensure the robustness of the experiment.
2.3 Serum samples
Convalescent serum samples were collected from patients with SARS-CoV-2 infection confirmed using real-time RT-PCR testing of nasopharyngeal swabs. The SARS-CoV-2 antibody in the serum was determined using an indirect ELISA, which indicates whether the samples bind specifically to the SARS-CoV-2 S protein and is considered the reference serological test for CoV. Stored serum samples collected from five healthy subjects at the Guangzhou Women and Children's Medical Center before 2019 were used as controls. Specimens were stored at 20°C until use, and written informed consents were obtained from the study subjects. The study protocol was reviewed and approved by The First Affiliated Hospital of Guangzhou Medical University Ethics Committee (No. 2020-77). Parts of the study were conducted to improve the quality of experiments and were therefore exempted from review.
2.4 ELISA
Indirect ELISA was performed as described in a previous study, with some modifications.16 Convalescent serum samples from patients with COVID-19 were used to confirm the epitopes detected using ELISA. Briefly, 96-well ELISA plates were coated with synthesized conjugated peptides (4 μg/ml) overnight at 4°C in carbonate-buffered saline (pH 9.6). Afterward, serum samples were added to the respective wells at 1:200 dilution and then incubated at 37°C for 1 h. After washing four times, the plates were incubated with a 1:5000 dilution of horseradish peroxide (HRP)-conjugated goat anti-human IgG secondary antibody (Sigma-Aldrich) at 37°C for 1 h, and then TMB substrate was added to develop color. The reaction was stopped with 2 M H2SO4, and then the absorbance values of the plate were determined.
Competitive inhibition ELISA was performed to confirm the epitopes detected by the MAbs.16 The optimized concentrations of the MAbs were determined using serial dilution. Briefly, recombinant SARS-CoV-2 NP in carbonate-bicarbonate buffer (pH 9.6) were coated onto 96-well ELISA plates overnight at 4°C and then blocked. Increasing concentrations (0.5, 1, 2, 5, 50, or 100 μg/ml) of peptides were added to the diluted MAbs in the blocking buffer and then incubated for 1 h at 37°C. MAbs without peptides (0 μg/ml) were used as controls. Next, the MAb-peptide mixtures were added to the coating plate and incubated for 1 h at 37°C. The experiment was then performed according to the protocol described above.
A double-antibody sandwich ELISA was established using the MAbs and a RAb previously developed.16 Briefly, ELISA plates were coated with RAb (1:500; Sino Biological Inc.) in phosphate-buffered saline (PBS) (pH 7.4) overnight at 4°C and then blocked with a blocking buffer. Increasing concentrations (0, 0.01, 0.1, 1, or 10 μg/ml) of purified SARS-CoV-1 NP, SARS-CoV-2 NP, and HCoV-OC43 NP were added to the respective wells in the blocking buffer and incubated at 37°C for 1 h. The MAbs (K1-32, K1-92, and K1-175; 1:1000 dilution) were then added to the plate after washing. Subsequently, an HRP-conjugated goat anti-mouse IgG secondary antibody was added. The experiment was then performed using the protocol described above.
2.5 Western blot analysis
Western blot analysis was performed as described previously.16 Briefly, protein samples were denatured at 98°C for 5 min, separated using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and then electrophoretically transferred onto nitro-cellulose membranes. The membranes were blocked with 5% skim milk in PBS and then reacted with MAbs at a final dilution of 1:6000. The membranes were washed and then incubated with HRP-conjugated goat anti-mouse IgG secondary antibody at a final dilution of 1:6000. Afterward, 1-Step Ultra TMB Blotting Solution substrate (Thermo Fisher Scientific) was added to develop the blots.
2.6 Multiple sequence alignments of NPs from HCoVs and SARS-related CoVs
SnapGene 4.2.4 was used to perform routine sequence management. MAFFT was used to perform sequence alignment with default parameters. We retrieved the following protein sequences from the National Center for Biotechnology Information (NCBI) database: HCoV-HKU1 (NC_006577), HCoV-OC43 (NC_005147), HCoV-229E (NC_002645), HCoV-NL63 (NC_005831), SARS-CoV-2 (NC_045512), SARS-CoV-1 (NC_004718), MERS-CoV (NC_019843), SARSr-CoV RaTG13 (MN996532.2), SARSr-CoV BtKY72 (KY352407.1), and SARSr-CoV PC4-227 (AY613950.1). The following SARS-CoV-2 variants were retrieved from GenBank: Alpha (B.1.1.7) [MZ314997.2], Beta (B.1.351) [MZ433432.1], Gamma (P.1) [MZ427312.1], Eta (B.1.525) [MZ362451.1], Iota (B.1.526) [MZ702431.1], Kappa (B.1.617.1) [MZ571142.1], Zeta (P.2) [MZ833438.1], Delta (B.1.617.2) [OK091006.1], Epsilon (B.1.427) [MW424864], Epsilon (B.1.429) [MW306426], Mu (B.1.621) [MZ233528], Omicron (BA.1) [OP002912], Omicron (BA.2) [OP081448], Omicron (BA.3) [ON330833], Omicron (BA.4) [BS004860], and Omicron (BA.5) [OP107818].
2.7 Homology modeling of SARS-CoV-2 NP
The three-dimensional (3D) structure of the SARS-CoV-2 NP was predicted using the Robetta server (University of Washington), which provides automated tools for the prediction and analysis of the tertiary structures of proteins.17 The model was optimized using the TrRefineRosetta modeling method.18 In addition, the 3D crystal structure of SARS-CoV-2 NP (Protein Data Bank [PDB] ID: 6M3M, 6YUN) was downloaded from the PDB database for comparison with the modeled 3D structure of the SARS-CoV-2 NP. The Robetta server then predicted the tertiary structure of a particular protein based on input genomic data, and the surface localization of each epitope was determined using the PyMOL3.9 software.
3 RESULTS
3.1 Production and epitope mapping of developed MAbs
A total of 141 MAbs obtained after mice immunization were diluted and reacted with prepackaged SARS-CoV-2 NP, of which 90 MAbs with high reactivity and affinity for SARS-CoV-2 NP were preliminarily screened for further analysis (Figure 1A).
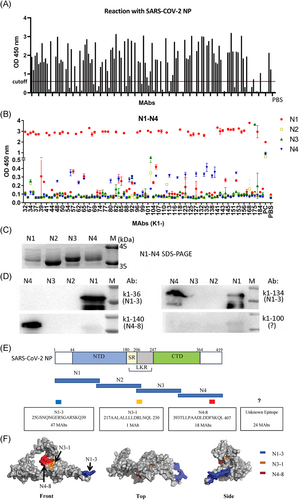
Four truncated fragments overlapping the full-length SARS-CoV-2 NP, namely, N1, N2, N3, and N4, were used to identify the region bound with MAbs. Of the 90 MAbs, 47 reacted with SARS-CoV-2 NP-N1, 18 reacted with SARS-CoV-2 NP-N4, and only one MAb (K1-175) reacted with SARS-CoV-2 NP-N3 (Figure 1B). Figure 1C showed the SDS-PAGE result of the four recombinant fragment proteins. Consistent with the ELISA results, the western blot showed that MAb K1-36 bound to only N1, MAb K1-140 bound to N4, and MAb K1-100 did not bind to any fragment (Figure 1D). The weak bands showing N1 bound to MAb k1-140 and k1-100 may be due to nonspecific bindings. Interestingly, K1-134 bound to N1 and N4, and ELISA results revealed that K1-134 strongly bound to N1 but weakly bound to N4. Significant regional dominance was not observed in the remaining 24 MAbs, and each region of SARS-CoV-2 NP responded weakly or did not respond at all. Therefore, these 24 MAbs may detect discontinuous epitopes.
In addition, each region of SARS-CoV-2 NP was subdivided into nine short overlapping peptides (Supporting Information: Table S1). Competitive inhibition and indirect ELISA results indicated that 47, 18, and 1 (K1-175) MAbs bound to N1-3 (25GSNQNGERSGARSKQ39), N4-8 (393TLLPAADLDDFSKQL407), and N3-1 (217AALALLLLDRLNQL230), respectively (Figure 1E; Table 1).
| Epitope | Amino acid sequence | MAbs (titer) | ||
|---|---|---|---|---|
| >106 | 106–105 | 105–104 | ||
| N1-3 | 25GSNQNGERSGARSKQ39 | K1-32, K1-34, K1-36, K1-41, K1-47, K1-49, K1-68, K1-77, K1-107, K1-108, K1-118, K1-126, K1-131, K1-141, K1-142, K1-145, K1-150, K1-151, K1-153, K1-155, K1-156 | K1-37, K1-38, K1-42, K1-50, K1-59, K1-64, K1-67, K1-69, K1-75, K1-78, K1-82, K1-85, K1-86, K1-96, K1-97, K1-101, K1-124, K1-125, K1-134, K1-143, K1-168 | K1-33, K1-54, K1-102, K1-111, K1-179 |
| N3-1 | 217AALALLLLDRLNQL230 | K1-175 | ||
| N4-8 | 393TLLPAADLDDFSKQL407 | K1-90, K1-92, K1-140, K1-152, K1-135 | K1-48, K1-55, K1-65, K1-83, K1-94, K1-105, K1-113, K1-117, K1-123, K1-127, K1-138 | K1-62, K1-80 |
| Undefined | Unknown | K1-71, K1-99, K1-163 | K1-39, K1-44, K1-57, K1-66, K1-81, K1-88, K1-119, K1-132, K1-137, K1-144, K1-171, K1-184 | K1-40, K1-51, K1-74, K1-100, K1-104, K1-110, K1-115, K1-116, K1-186 |
Since the structure of the full-length NP is yet to be elucidated, we built an NP model using the Robetta online server. Molecular modeling of SARS-CoV-2 NP showed that N1-3 and N4-8 were located on the surface of the protein, while N3-1 was partially located on the surface of the protein and immediately adjacent to N4-8. In the 3D structure, the N1-3 region was separated from the two other epitopes (Figure 1F).
3.2 Cross-reaction of MAbs with other HCoV NPs
Notably, all MAbs recognizing epitopes N3-1 and N4-8, as well as 24 MAbs targeting unidentified epitopes, cross-reacted with SARS-CoV-1 NP, whereas only one of the 47 MAbs (K1-134) conjugated to N1-3 cross-reacted with SARS-CoV-1 NP (Supporting Information: Figure S1A). None of the 90 MAbs reacted with HCoV-NPs (HCoV-229E, HCoV-OC43, HCoV-HKU1, HCoV-NL63) and MERS-CoV NPs using ELISA (data not shown).
Purified NPs of the seven CoVs transmissible between human beings were prepared and tested using SDS-PAGE (Supporting Information: Figure S1B,C). MAb K1-134 bound to SARS-CoV-2 and SARS-CoV-1 NPs by western blot, while the remaining MAbs that recognized the N1-3 epitope, such as K1-36, bound only to SARS-CoV-2 NP. On the other hand, MAb K1-140 that recognized the N4-8 epitope and MAb K1-110 that recognized an unknown epitope bound to both SARS-CoV-2 and SARS-CoV-1 NPs. None of the MAbs reacted with NPs of HCoV-229E, HCoV-OC43, HCoV-HKU1, HCoV-NL63, and MERS-CoV (Supporting Information: Figure S1C). A weak band was observed for HCoV-HKU1 NP bound to MAb K1-110, which may indicate a nonspecific reaction.
3.3 Similarity analysis of the epitopes of SARSr-CoVs and SARS-CoV-2 variants
SARS-CoV-2, SARS-CoV-1, SARS-CoV-PC4-227, SARSr-CoV RaTG13, and SARSr-CoV-BtKY72 are members of SARSr-CoV.19, 20 Multiple alignments of NPs derived from five SARSr-CoV strains and five HCoVs were performed to elucidate the distribution of the epitope regions. SARS-CoV-2-N1-3, SARS-CoV-N4-8, and SARS-CoV-N3-1 were unique from the other five HCoVs (HCoV-229E, HCoV-OC43, HCoV-HKU1, HCoV-NL63, and MERS-CoV) (Figure 2A). The N1-3 epitope differed from other HCoVs by >5 amino acids, while the N3-1 and N4-8 epitopes differed from those of SARS-CoV-1 NP, SARSr-CoV PC4-227 NP, SARSr-CoV BtKY72 NP by only one amino acid, thus allowing MAb cross-reactivity with SARS-CoV-1. It is noteworthy that the SARS-CoV-2-N4-8 and -N3-1 epitopes were highly conserved in SARSr-CoVs. The corresponding regions of N1-3, N4-8, or N3-1 epitopes in SARS-CoV-1 and SARSr-CoV PC4-227 were not different (Figure 2A); the corresponding regions of N3-1 epitope in SARS-CoV-1 and SARSr-CoV BtKY72 were also not different. As the cross-reaction of MAbs with SARS-CoV-1 was evaluated using ELISA and western blot with recombinant SARS-CoV-1 NP, the corresponding peptides of SARS-CoV-1 were not synthesized for further investigation.
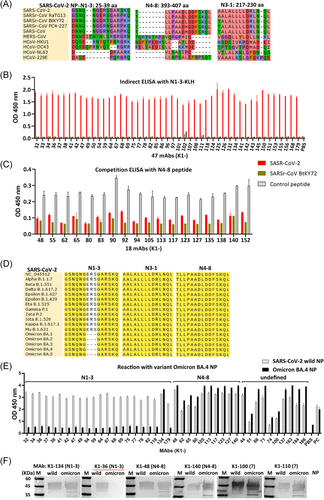
SARSr-CoV BtKY72 was not closely related to the other clustered SARSr-CoV strains. Six amino acids were found in the corresponding N1-3 epitope of SARSr-CoV BtKY72, which were not consistent with SARS-CoV-2. The N1-3 peptide of BtKY72 strain was then synthesized, and results showed that it could not be detected by all 47 MAbs that identified the N1-3 epitope (Figure 2B). Of the 47 MAbs, 46 MAbs did not react with SARS-CoV-1. These results indicate that 46 MAbs are highly specific to SARS-CoV-2, and the N1-3 epitope is highly specific to SARS-CoV-2.
One amino acid (D399E) was observed in the corresponding N4-8 epitope of SARSr-CoV BtKY72, which was not consistent with other SARSr-CoVs. This peptide was then synthesized, and it was found that the peptide could be recognized by all 18 MAbs that identified the N4-8 epitope (Figure 2C). This result indicates that 18 MAbs may broadly detect all SARSr-CoV strains, and D399E may not be a critical amino acid for binding.
Furthermore, the N4-8 and N3-1 epitopes were completely conserved in all circulating mutants of SARS-CoV-2, including Alpha, Kappa, Delta, and the current Omicron variants, BA.2, BA.4, and BA.5. No amino acid mutation was found in these regions (Figure 2D). Strikingly, all Omicron variants have ERS deletion (E31, R32, S33) in the N1-3 epitope region compared to the reference strain (wild type), while the other variants of concern have no mutation in this region (Figure 2D). Indirect-ELISA showed that all MAbs targeting N4-8 and an undefined epitope have significant cross-reaction with BA.4 NP, and none of the 46 MAbs targeting N1-3 cross-reacted with BA.4 NP. Interestingly, K1-134 (N1-3) could detect BA.4 NP, which cross-reacts with SARS-CoV-1 NP (Figure 2E). The western blot results were consistent with the ELISA results (Figure 2F), indicating that ERS is important for the detection of the 46 MAbs targeting the N1-3 epitope, and the MAbs could not be used for detecting the current Omicron variants.
3.4 Identification and epitope recognition using human convalescent plasma
Next, whether the antibodies in anti-SARS-CoV-2 NP sera specifically recognize epitopes was analyzed via mice immunization, followed by sera collection and cross-reaction with the pre-encapsulated epitopes. Consistent with the MAb recognition results, N1-3 was the superior epitope, followed by N4-8 (Figure 3A).
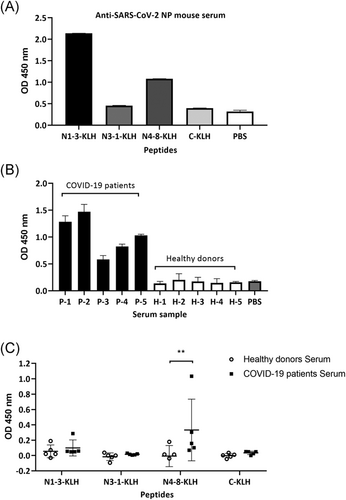
Furthermore, serum samples from five convalescent patients with COVID-19 and five healthy individuals were collected. Importantly, the presence of anti-SARS-CoV-2 antibodies in serum samples of patients with COVID-19 was identified by the binding of the serum to the SARS-CoV-2 spike protein (Figure 3B). Subsequently, the SARS-CoV-2 N1-3, N3-1, and N4-8 peptides, the control peptide (adenovirus 100K) coupled to KLH, and PBS as the coating control, were subjected to immune recognition reactions with the convalescent plasma, healthy plasma, and PBS (blank) samples, respectively. Antibodies against N4-8 peptide were significantly higher in the plasma of patients recovering from COVID-19 compared to healthy subjects (p < 0.01) (Figure 3C).
3.5 Establishment of double-antibody sandwich ELISA methods to quantify SARS-CoV-2 NP
Double antibody sandwich ELISA methods were established to further confirm that the MAbs are potential diagnostic tools. The selected MAbs simultaneously underwent reaction with the quantified NPs of SARS-CoV-2, HCoV-OC43, and SARS-CoV-1 (0.01–10 μg/ml). The MAbs did not bind to HCoV-OC43 NP, moreover, the OD values remained low and slightly fluctuated than those of SARS-CoV-2 NP or SARS-CoV-1 NP, regardless of concentration (Figure 4A–D). The unpurified SARS-CoV-2-NP was quantified using the sandwich methods. Cultured samples of human adenoviruses, human respiratory syncytial virus A, influenza virus A (PR8), HCoV-OC43, and HCoV-229E remained undetected by these methods (data not shown).
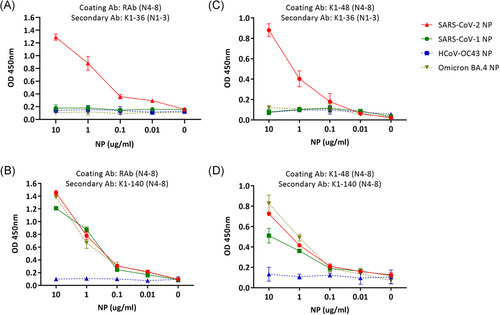
RAb, which was prepared previously, was used as the coating antibody. This resulted in K1-32 MAb to specifically bind to SARS-CoV-2 NP; however, not to SARS-CoV-1 NP. Moreover, the OD value decreased along with SARS-CoV-2 NP concentration. Therefore, MAb K1-32 and majority of the MAbs recognizing the N1-3 epitope could be used to specifically diagnose COVID-19 and distinguish SARS-CoV-2 from SARS-CoV-1 and other HCoVs (Figure 4A). In contrast, the MAb K1-140 targeting N4-8 epitope bound not only to SARS-CoV-2 NP but also to SARS-CoV-1 NP, and the decrease in OD values varied more significantly between 1 and 0.1 μg/mL of NP (Figure 4B). A sandwich ELISA was also established using mouse MAb pairs as coating antibody and secondary antibody (Figure 4C,D). The results were consistent with that using RAb as the coating antibody. Additionally, sandwich-ELISA was performed to recognize the Omicron variant. As expected, MAb K1-36 (N1-3) as the secondary Ab could not recognize omicron BA.4 NP, while MAb K1-140 (N4-8) as the secondary Ab could recognize omicron BA.4 NP. Therefore, the sandwich ELISA using a pair of MAbs recognizing N4-8 could be a useful tool to distinguish SARSr-CoVs from other HCoVs and help detect new SARSr-CoV strains. Furthermore, this method could be used to recognize the current circulating Omicron variants.
4 DISCUSSION
Early, rapid, and accurate diagnosis of COVID-19 plays a key role in the control of its transmission and may impact its treatment.21, 22 Serological tests cannot be used as an early diagnostic method because of the “window period” for the production of virus-specific antibodies, but they can complement molecular diagnosis. Owing to the limitations of the RT-PCR test, rapid and accurate point-of-care diagnostic tests for detecting viral antigens, such as an antigen-capture sandwich ELISA and immunochromatographic strip, may be advantageous for mass-screening of potential SARS-CoV-2 infected cases.23-26
In this study, 90 MAbs with high reactivity and affinity for SARS-CoV-2 NP were screened. The MAbs have no cross-reaction with common HCoVs (HCoV-229E, HCoV-OC43, HCoV-HKU1, HCoV-NL63) and MERS-CoV. Forty-four MAbs cross-reacted with SARS-CoV-1 NP, and other 46 MAbs have no cross-reaction with SARS-CoV-1. These MAbs could be a candidate tool library for proposing rapid and reliable antigen testing methods. Subsequently, an ELISA diagnostic method for SARS-CoV-2 antigen capture was established by using the recognition characteristics of MAbs. This method may be used for vaccine quantification or clinical SARS-CoV-2 diagnostics. Furthermore, this method showed the potential role of MAbs as diagnostic tools and proved the high specificity of the MAbs detecting SARS-CoV-2. As majority of the MAbs recognized the N1-3 epitope, this observation could be considered as a useful tool for distinguishing SARS-CoV-2 from SARS-CoV-1 and other HCoVs. Unfortunately, the MAbs recognizing the N1-3 epitope could not detect Omicron variants due to the deletion of ERS in N1-3 region. Nevertheless, the other MAbs could be used to develop a diagnosis method for distinguishing SARSr-CoV from common HCoVs and help identify novel members of SARSr-CoVs. In addition, the combination of RAb and a mouse MAb yielded a better result in the sandwich-ELISA method.
Information on NP epitope aids in exploring the structure and diagnosis. In this study, three B cell epitopes of NP (N1-3, N3-1, and N4-8) were identified using the MAbs. The N4-8 epitope was previously identified using a RAb, whereas N1-3 and N3-1 are novel epitopes. The main domain structures of SARS-CoV-2 NP consists of three distinct parts: an N-terminal RNA-binding domain (NTD), a C-terminal dimerization domain (CTD), and an intrinsically disordered central serine/arginine (SR)-rich linker region (LKR), while the two ends are internally disordered regions (IDR).7, 25, 27 The NTD and CTD are the functional domains of the virus nucleocapsid. However, the epitopes reported in this study are located in the LKR and IDR of NP. The LKR contains the N3-1 epitope that is tolerant of mutations.28 In addition, the IDR contains the N4-8 that is important in the regulation of NPs; it contributes to independent self-association to regulate the tetramer formation of NPs and mediates a homotypic interaction between NPs.28, 29 However, few reports on the study of two tails are available; therefore, MAbs targeting N4-8 and N1-3 can be exploited to study the structure and function of N protein in the IDRs.
SARS-CoV-2 NP epitopes stimulate the immune system to release various cytokines through helper T-lymphocytes and antigen-presenting cells, leading to the activation of cytotoxic T-lymphocytes and B-cell lymphocytes to lyse infected cells and kill pathogens. Based on immunoinformatics and epitope information from the Immune Epitope Database and Analysis Resource, N1-3 and N4-8 are B-cell epitopes, while the N3-1 epitope is a T-cell epitope that can recognize MHC.30-35 In the present study, N3-1 was also identified as a B cell epitope detected by MAb K1-175.
A previously reported RAb also recognized the N4-8 epitope of SARS-CoV-2 NP.16 Results indicated that the residues 393–407 of the NP is a B cell epitope in mice, rabbits, and humans. The MAbs targeting the N4-8 epitope captured not only SARS-CoV-2 NP but also SARS-CoV-1 NP. Furthermore, they were able to bind to SARSr-CoV-BtKY72. Therefore, 339D may not be a critical amino acid for these mouse MAbs. In contrast, 339D was a key amino acid in a previously studied RAb.16 A recent study also developed an antigen-capture sandwich ELISA using MAb targeting SARS-CoV-2 N4-8.25
In the present study, most of the MAbs recognized the N1-3 epitope, located on the surface of the protein in the molecular model. The N1-3 epitope has excellent parameters, such as high antigenicity, non-allergenicity, non-toxicity, hydrophilicity, flexibility, and beta-turn. Four of its amino acids (36RSKQ39) have surface accessibility and are likely to be readily recognized by MAbs.32, 34 As expected, N1-3 is also the dominant epitope identified in the murine anti-SARS-CoV-2 NP sera; therefore, it can be considered for serological testing and multi-epitope vaccine design. Unfortunately, the current epidemic omicron strains, including BA.1 to BA.5, exhibited ERS deletion in the N1-3 region, due to which many antibodies were unable to identify the omicron NP. These results indicate that antibody-based diagnostics may be ineffective against the new variants, although SARS-CoV-2 NP is relatively conserved. There was no mutation in N4-8 and N3-1 regions for all current variants of concern, demonstrating the importance of the MAbs detecting these two regions.
Likewise, a difference in the identified immunodominant epitopes was observed between murine-derived anti-SARS-CoV-2 sera and sera derived from patients with COVID-19. The N4-8 was the immunodominant epitope in human beings, whereas, N1-3 was the immunodominant epitope in mice. In the early studies of SARS-CoV-1, the dominant epitopes identified in the anti-SARS-CoV-1 sera from mice and monkeys did not correspond to those identified in sera from patients infected with SARS-CoV-1, even though all share the same epitopes.36 The B cell epitope in the same location as N4-8 was identified using serum derived from SARS-CoV-1-infected individuals. This may be due to differences in major histocompatibility complex across species and individuals, or due to live virus infection rather than immunization with purified protein in humans.
Finally, three epitopes were identified using the MAbs from immunized mice. Majority of previously reported B-cell epitopes of SARS-CoV-2 NP were identified using plasma or serum of patients with COVID-19 (http://www.iedb.org/), and only one study reported two epitopes using the MAbs from a COVID-19 convalescent patient.8 Currently, many linear B-cell epitopes of SARS-CoV-2 NP have been identified (http://www.iedb.org/), but only one discontinuous epitope recognized by an antibody was reported.8 The detected epitope was not identified in the 24 MAbs; therefore, they may recognize a discontinuous epitope of NP. These MAbs may need to bind to the specific spatial structure of the SARS-CoV-2 NP, as the epitope is present in both linear and spatial conformations.31 Therefore, further studies should be done to reveal the epitopes detected by these MAbs.
This study has some limitations. First, due to the strict biosafety restrictions of the SARS-CoV-2 experiments, the MAbs could only be assessed using recombinant NP and not using the live virus. Future studies should evaluate the MAbs using clinical SARS-CoV-2 samples. Second, for the remaining 24 MAbs that do not bind to any region, further study is a requirement to clarify the binding site using several technical methods, such as epitope mutation and electron microscopic observation of antibody-NP complexes. Third, serum samples from a relatively small number of donors were used to identify epitopes. A larger sample group should be used to further determine epitope dominance and provide a clinical basis for designing vaccines and antigen testing. Finally, 46 MAbs detecting the N1-3 epitope could not react with the Omicron NP. Therefore, these MAbs are not able to detect/study the dominant SARS-COV-2 variants currently circulating globally. However, using these MAbs a method to detect the dominant SARS-COV-2 variants currently circulating globally from other circulating SARS-CoV-2 strains can be established.
In summary, several MAbs that could distinguish SARS-CoV-2 from common HCoVs or identify novel SARSr-CoVs were examined. Due to their high specificity and high titer, the MAbs can serve as good candidate reagents for antigen detection kits for COVID-19, showing promising clinical applications. Three B cell epitopes, namely N1-3, N3-1, and N4-8, were mapped in the LKR and IDR. This study provided a basis for auxiliary antigen assays of SARS-CoV-2.
AUTHOR CONTRIBUTIONS
Xingui Tian planned and designed the study. Xingui Tian and Liling Zhou wrote the manuscript. Xingui Tian, Liling Zhou, Yujie Yang, Chuncong Mo, Zhichao Zhou, Aiping You, Ye Fan, Wenkuan Liu, and Xiao Li performed the experiments. Xingui Tian and Rong Zhou guided the study. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
This study was supported by National Natural Science Foundation of China: (grant number 82072264); the Guangzhou Science and Technology Program co-funded by Zhongnanshan Medical Foundation of Guangdong Province (202102010359, 202102010364, and ZNSA-2020003); Natural Science Foundation of Guangdong Province, China: (grant number 2021A1515011071); Emergency Key Program of Guangzhou Laboratory (EKPG21-13); Special Project for COVID-19 Prevention and Control of Zhongnanshan Medical Foundation of Guangdong Province (ZNSA-2020012); and the Guangdong-Hong Kong-Macao Joint Laboratory of Respiratory Infectious Disease (GHMJLRID-Z-202109).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




