High titers of neutralizing antibodies in the blood fail to eliminate SARS-CoV-2 viral RNA in the upper respiratory tract
Lu Li, Jianping Cui, Jingyan Tang, and Jingrong Shi contributed equally to this study.
Abstract
Retest-positive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) viral RNA, as a unique phenomenon among discharged individuals, has been demonstrated to be safe in the community. Still, the underlying mechanism of viral lingering is less investigated. In this study, first, we find that the frequency of viral RNA-positive retesting differs among variants. Higher ratios of viral RNA-positive retest were more frequently observed among Delta (61.41%, 514 of 837 cases) and Omicron (39.53%, 119 of 301 cases) infections than among ancestral viral infection (7.27%, 21 of 289 cases). Second, the tissues where viral RNA reoccurred were altered. Delta RNA reoccurred mainly in the upper respiratory tract (90%), but ancestral virus RNA reoccurred mainly in the gastrointestinal tract (71%). Third, vaccination did not reduce the frequency of viral RNA-positive retests, despite high concentrations of viral-specific antibodies in the blood. Finally, 37 of 55 (67.27%) Delta-infected patients receiving neutralizing antibody therapy become viral RNA retest positive when high concentrations of neutralizing antibodies still patrol in the blood. Altogether, our findings suggest that the presentence of high titers of neutralizing antibodies in the blood is incompetent in clearing residual viral RNA in the upper respiratory tract.
1 INTRODUCTION
Severe acute respiratory syndrome coronavirus (SARS-CoV-2) infection affected over 600 million cases worldwide as of September 2022.1 As a unique phenomenon, SARS-CoV-2 viral RNA becoming positive retest after discharge, the criteria of which is two consecutive viral RNA test negative at an interval of >24 h, was firstly reported at the very beginning of the SARS-CoV-2 pandemic2 and was subsequently observed at a frequency from 3.3% to 7.3%3-5 for ancestral SARS-CoV-2 infection. Recently, we reported that Delta infection resulted in a significantly higher frequency of viral RNA retest positive (approximately 60%),6 suggestive of a correlation of more frequent occurrence of viral RNA retest positive with novel variants with higher viral titers. The status of viral RNA reoccurrence in Omicron infection is less reported. The virus and host immune responses are postulated to be two main factors contributing to viral RNA-positive retesting.
In one aspect, novel mutations or reinfections are suspected of causing the positive retest of viral RNA. As of the origin, the retest-positive viral RNA is phylogenetically identical to the initial virus but not a reinfection3, 7, 8 (also unpublished data). Also, no novel mutation is found to cause the viral RNA reoccurrence. We have reported that the SARS-CoV-2 viral RNA concentrations during the repositive stage are about 10e5–10e6 times lower than during the first hospitalization, and the alive virus, if any left, is barely infectious and unlikely to cause community transmission.6
In the other aspect, the host immune status is postulated to account for the viral RNA reoccurrence. An immunocompromised state is associated with a high frequency of SARS-CoV-2 persistence.9 We did observe a delayed and compromised viral-specific IgM, IgA, and IgG generation in the ancestral SARS-COV-2 infection.3 The relation between viral reoccurrence and viral-specific immune response for novel variants warrants further investigation.
In this study, we aim to investigate whether an increased immune response could reduce the recurrence of viral RNA. On the one hand, a prior vaccination will actively facilitate the quick adaptive immune response when a breakthrough infection occurs. On the other hand, passive infusion of viral-specific neutralizing antibodies can directly provide an immune barrier against viral infection and replication.
2 METHODS
2.1 Patient information
Patients infected with the SARS-CoV-2 Delta variant include 158 local cases from May 21 to June 18, 2021, and 679 imported patients from July 1 to November 24, 2021.10 Patients infected with the Omicron variant include 301 cases from admission on December 12, 2021 (the first case) to discharge in February 2022. In addition, 289 patients infected with wild-type SARS-CoV-2 were included, as previously reported.3 We analyzed the vaccine effects and neutralizing antibody treatment on positive retesting in Delta-infected patients. When analyzing the vaccine effect on viral RNA positive retesting, we excluded patients who received a neutralizing antibody therapy or one-dose vaccination, with 716 cases remaining (Supporting Information: Figure 1). When analyzing the effect of neutralizing antibody therapy, we excluded all vaccinated individuals and those receiving convalescent plasma and retained 365 patients. This study was approved by Guangzhou Eighth People's Hospital Ethics Committee (Nos. 202115202 and 202113200). Written informed consent was obtained from all patients. All diagnostic and discharge criteria were according to the Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia published by the Chinese National Health Commission (Trial version 7 for wild-type SARS-CoV-2 and Trial version 8 for Delta and Omicron variants).11 All patients who fulfilled the discharge criteria were suggested for another 14-day quarantine to monitor their health. Viral RNA is also tested by reverse transcription polymerase chain reaction (RT-PCR) in this quarantine period. This study defines a viral RNA-positive retest as detectable viral RNA by RT-PCR after discharge.
2.2 Clinical data collection
The demographic profiles, clinical characteristics, vaccination history, and RBD-IgG titers of coronavirus disease 2019 (COVID-19) patients were collected from the hospital and laboratory information systems. Serum IgG antibodies against the SARS-CoV-2 RBD spike protein were tested with two-step indirect immunoassay electrochemiluminescence immunoassay kits (Antu Biotech Co., Ltd.) as reported.11
2.3 Viral RNA detection and sequencing
The detailed protocol for sample collection and viral RNA detection is as previously described.3, 10 Briefly, the swab samples were collected according to standardized procedures. Viral RNA extraction and RT-PCR detection were performed with Nucleic Acid Isolation Kit and RNA Detection Kit (Da'an Gene Co. Ltd.; Cat. Nos.: DA0630 and DA0930) according to the manufacturer's instructions. Positive results were determined if a cycle threshold value (Ct value) was less than 40 (200 copies/ml) either for N or ORF1a/b gene.
Virus subtyping was performed by whole viral genome sequencing using the next-generation sequencing as previously described.10
2.4 Messenger RNA-sequencing
The peripheral blood mononuclear cell (PBMC) transcriptome data from our previous publication11 were reanalyzed using similar strategies but were based on viral RNA-positive retest.
2.5 Neutralizing antibody therapy
A clinical trial (Clinical trial no. 202113200) of BRII-196 plus BRII-198 injection (Brii Biosciences) was carried out in our hospital from May to September 2021 when Delta variant of concern was epidemic. This trial is a one-armed, open, prospective study selecting COVID-19 patients to receive a single dose of BRII-196 plus BRII-198. The enrolled subjects immediately received 1000 mg of BRII-196, followed by 1000 mg of BRII-198 by intravenous infusion. This study analyzed the retest viral RNA of 66 COVID-19 patients included in the clinical trial.
2.6 Statistical analysis
The data were statistically analyzed by SPSS software. Student's t-test (for independent continuous variables with normal distribution) and χ2 test (for categorical variables) were used to analyze the differences between the two groups. Kruskal–Wallis H-test with post hoc pairwise comparisons with Bonferroni adjustment was used for multiple comparisons. When p < 0.05, differences were deemed statistically significant.
3 RESULTS
3.1 SARS-CoV-2 viral RNA-positive retest differs among variants
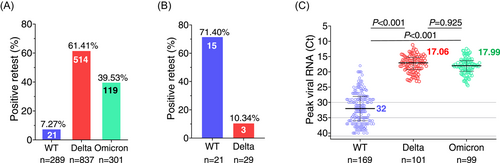
We compared the positive retest SARS-CoV-2 viral RNA among different variants. In 2020, 21 of 289 individuals (7.27%) infected with the ancestral SARS-CoV-2 had reoccurred viral RNA (Figure 1A). However, frequencies of viral RNA-positive retest in Delta and Omicron infection increased substantially to 61.41% (514 of 837 cases) and 39.53% (119 of 301 cases), respectively. Ancestral SARS-CoV-2-infected patients are more likely to have detectable retest viral RNA in the anal swabs (15 of 21 cases, 71.40%) (Figure 1B), consistent with the observation that ancestral SARS-CoV-2 infection leads to viral RNA persistence in the gastrointestinal tract for 7 months.7, 8 In contrast, the viral RNA-positive retest in anal swabs significantly decreased to 10.34% (3 of 29 cases) for Delta virus infection. Of note, the peak viral titers in nasopharyngeal or oropharyngeal swabs of Delta (median Ct = 17.06) and Omicron (median Ct = 17.99) (both variants are equal without a significant difference) are substantially higher than that of ancestral virus (median Ct = 32) (Figure 1C), indicating that high viral concentrations might be one key contributor to viral RNA reoccurrence in the upper respiratory tract.
3.2 High concentrations of viral-specific antibodies in Delta breakthrough infection can not prevent viral RNA reoccurrence
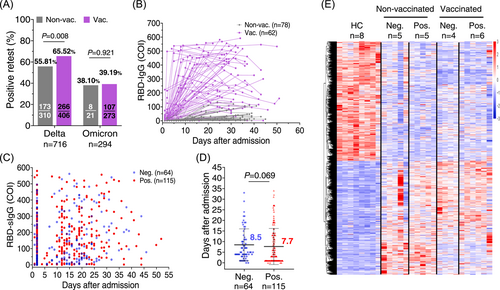
Next, we evaluated the serum antibody in the patients with positive retest viral RNA. In vaccinated individuals, the breakthrough infection can effectively recall the pre-existing B-memory cells and prompt the generation of high titer viral-specific antibodies, contributing to an expedited virus suppression.11-14 Of 406 Delta variant-infected and fully vaccinated (at least two doses) patients, 266 (65.52%) became viral RNA retest positive, and 107 of 273 fully vaccinated (at least two doses) patients infected with Omicron variant did (39.19%) (Figure 2A and Table 1). Then, we analyzed the antibody kinetics of Delta variant-infected individuals. The RBD-IgG antibody levels were significantly higher in the breakthrough infection than in the vaccine-naïve patients (Figure 2B). However, patients with positive retest viral RNA have similar levels of RBD-specific antibodies to those without positive retest viral RNA (Figure 2C). Additionally, we observed a similar trend of viral RNA decline to undetectable levels between viral RNA retest-positive (median = 7.7 days) and viral RNA retest-negative (median = 8.5 days) groups during the first hospitalization (Figure 2D).
| Two-dose vaccination (n = 179) | p value | Neutralizing antibody therapy (n = 55) | p Value | |||
|---|---|---|---|---|---|---|
| Neg. (n = 64) | Pos. (n = 115) | Neg. (n = 18) | Pos. (n = 37) | |||
| Sex, n (%) | ||||||
| Male | 54 (84.38) | 85 (73.91) | 0.107 | 9 (50.00) | 21 (56.76) | 0.637 |
| Female | 10 (15.62) | 30 (26.09) | 9 (50.00) | 16 (43.24) | ||
| Age, years (mean ± SD) | 39.91 ± 10.83 | 37.3 ± 10.85 | 0.125 | 54.72 ± 19.63 | 51.49 ± 17.60 | 0.540 |
| 14 < age ≤ 60, n (%) | 63 (98.44) | 114 (99.13) | 0.673 | 9 (50.00) | 25 (67.57) | 0.212 |
| >60, n (%) | 1 (1.56) | 1 (0.87) | 9 (50.00) | 12 (32.43) | ||
| Clinical classification, n (%) | ||||||
| Moderate | 25 (39.06) | 40 (34.78) | 0.569 | 11 (61.11) | 20 (54.05) | 0.624 |
| Mild | 39 (60.94) | 75 (65.22) | 7 (38.89) | 17 (45.95) | ||
| Comorbidities, n (%) | ||||||
| Hypertension | 5 (7.81) | 7 (6.09) | 0.896 | 3 (16.67) | 13 (35.14) | 0.157 |
| Chronic heart disease | 3 (4.69) | 0 (0) | 0.044a | 3 (16.67) | 1 (2.70) | 0.188 |
| Diabetes | 0 (0) | 6 (5.22) | 0.090 | 2 (11.11) | 4 (10.81) | >0.999 |
| Liver disease | 1 (1.56) | 7 (6.09) | 0.305 | 1 (5.56) | 1 (2.70) | >0.999 |
| Lung disease | 2 (3.13) | 2 (1.74) | 0.941 | 0 (0) | 3 (8.11) | 0.543 |
| Vaccine type, n (%) | ||||||
| Inactivated vaccine | 59 (92.19) | 91 (79.13) | 0.023a | - | - | - |
| mRNA vaccine | 0 | 7 (6.09) | 0.051 | - | - | - |
| Adenovirus vector vaccine | 0 | 2 (1.74) | 0.538 | - | - | - |
| Unknown | 5 (7.81) | 15 (13.04) | 0.287 | - | - | - |
| Days from the last vaccine dose (mean ± SD) | 140.89 ± 99.54 | 133.33 ± 109.72 | 0.655 | |||
| ≤90, n (%) | 28 (45.90) | 60 (53.10) | - | - | - | |
| 90 < day ≤ 180, n (%) | 12 (19.67) | 18 (15.93) | 0.433 | - | - | - |
| >180, n (%) | 21 (34.43) | 35 (30.97) | - | - | - | |
| Unknown | 3 (4.69) | 2 (1.74) | - | - | - | |
- Note: Data are presented as mean ± standard deviation (SD) and number (%). p Values were determined using Student's t-test for age, Mann–Whitney U-test for age distribution, clinical classification, and days from the last vaccine dose, and χ2 test for sex, comorbidities, and vaccine type. The two-dose vaccination group excluded individuals receiving NAb therapy and the NAb therapy group excluded the vaccinated individuals.
- Abbreviations: mRNA, messenger RNA; NAb, neutralizing antibody therapy; Neg., viral RNA retest negative; Pos., viral RNA retest positive.
- a p Value is statistically significant.
RNA-sequencing (RNA-seq) of PBMCs collected within 1-week post-Delta infection revealed that a prior vaccination substantially reduced the gene expression changes compared to the nonvaccinated individuals and subsided antiviral inflammation response.11 RNA-seq analysis also failed to identify obvious characteristic patterns associated with viral reoccurrence (Figure 2E). Patients with positive retest had increased SYCP2L and decreased SEMA6B messenger RNA (mRNA) expression compared with negative retest patients in the unvaccinated group. In the two-dose vaccinated group, patients with positive retest had decreased AL603783.1, CUBN, and PTGS2 mRNA expression compared with negative retest patients. The underlying mechanism is still under investigation. Therefore, robust immune response in the peripheral blood barely accelerated residual viral RNA clearance in the upper respiratory tract.
3.3 Therapeutic-neutralizing antibodies are unrelated to viral RNA reoccurrence
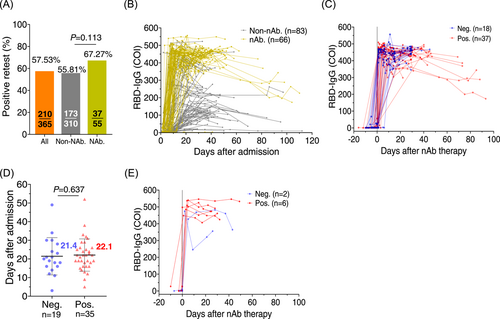
The actual viral replication suppression by the high concentrations of RBD-IgG in the breakthrough infection is questioned. To investigate whether high titers of neutralizing antibodies could reduce viral RNA reoccurrence, we analyzed the viral RNA-positive retest in a cohort of patients receiving broad neutralizing antibodies against Delta infection. Fifty-five unvaccinated Delta-infected patients in Guangzhou in 2021 received the BRII-196/198 neutralizing antibody combination (now approved by China FDA for clinical use) and 37 patients (67.27%) had viral RNA reoccurrence after discharge, at a ratio similar to that in the nonneutralizing antibody-treated and nonvaccinated controls (55.81%, n = 173) (Figure 3A and Table 1). Despite the high levels of RBD-IgG after neutralizing antibody treatment (Figure 3B), most of these patients still had viral RNA reoccurrence (Figure 3C). Meanwhile, the neutralizing antibodies were detected to maintain for over 3 months, excluding the possibility that viral reoccurrence was due to the fast vanishing of passively intravenous-infused antibodies (Figure 3C). We also observed that the median time for viral RNA to become negative during the first hospitalization was 21–22 days for viral RNA retest-negative and viral RNA retest-positive groups, respectively (Figure 3D), implying that the neutralizing antibody failed to expedite the viral RNA-negative group during the first hospitalization. In addition, seven Delta variant-infected individuals, who were both vaccinated with two doses of vaccines and received neutralizing antibody therapy, maintained extremely higher serum RBD-binding antibodies. Still, six had positive retest viral RNA (Figure 3E).
4 DISCUSSION
In this study, we observed that high levels of serum-neutralizing antibodies seem ineffective in clearing residual SARS-CoV-2 viral RNA in the upper respiratory tract. The titers of RBD-specific binding antibodies in blood could represent its neutralizing capacity.15 The full vaccination dose markedly benefits high titers of antibody generation when Delta variant breakthrough infection occurs (Figure 2B). Breakthrough infection means an increased number of exposures to SARS-CoV-2 antigens, which will recall the pre-existing cross-reactive B-cell memory and elicit superior neutralizing antibodies.12-14 Meanwhile, intravenous infusion of high concentrations of well-defined neutralizing antibody BRII-196/198 still could not prevent viral RNA reoccurrence (Figure 3). As known, the surface of the upper respiratory tract is a unique anatomical site responsible for air cleaning by trapping small foreign particles. Its mucosa is usually tolerant to avoid misfiring to frequently encountered foreign antigens. However, immune status in the lower respiratory tract, where gas exchange takes place, differs in that the endless supply of cytokines, antibodies, and cells to the alveoli from the constant bloodstream forms a barrier against pathogen intrusion. Therefore, breakthrough infection instantly activates the vaccination-primed immune memory, and high concentrations of therapeutic neutralizing antibodies infused in peripheral blood guarantee the lung mucosa to access plenty of viral-specific antibodies and cytotoxic lymphocytes, which built a lagged but still potent antiviral barriers in the mucosa to reduce the viral burden and related pneumonia. The antiviral supply from peripheral blood to the mucosa surface in the upper respiratory tract is severely inadequate to compensate for an ineffective local antiviral capacity, resulting in viral lingering and occasionally detection. Incapability to mount a robust immune response in the mucosa rather than in the peripheral blood where high titers of neutralizing antibody enriched are thus supposed to be the key reason for viral lingering in the upper respiratory tract.
On the other hand, we previously found that SARS-CoV-2 RNA can persist for many weeks in a noninfectious status (mostly fragmented viral genomic RNA) in the respiratory tract.6, 16 Our results in this study further add to our knowledge that robust immune response in the serum seems helpless in eliminating the residual viral RNAs. The underlying mechanisms still need further exploration.
The current study has the following limits. First, mucosal sample collection from the upper respiratory tract is technically challenging since it is very uncomfortable for patients. The nasopharyngeal swabs and nasal flushing procedures also highly rely on the experience and the fluid volume. Second, a reliable quantitative test for measuring viral-specific antibodies, such as secreted IgA and IgG, in the mucosa of the upper respiratory tract is absent. Third, since it is a retrospective analysis, viral-specific T-cell immunity in the blood is missing, and the sample size is not large enough in some subgroups.
In conclusion, our study found that high serum-neutralizing antibodies are ineffective in eliminating residual SARS-CoV-2 RNA in the upper respiratory tract. Mucosal immunodeficiency may be the key reason for viral lingering in the upper respiratory tract. Further exploring the underlying discrepancy between systematic and mucosal immune responses is warranted for a better vaccine design to control mucosa infection.
AUTHOR CONTRIBUTIONS
Lu Li, Jianping Cui, Jingyan Tang, and Jingrong Shi did the data collection, laboratory tests, and data analysis. Xilong Deng and Ying Liu managed the patients and performed the neutralizing antibody therapy. Xiaowen Zheng and Qinghong Fan analyzed the data and prepared the figures. Haisheng Yu, Xiaoping Tang, Fengyu Hu, and Feng Li designed and supervised the study. Lu Li and Feng Li wrote the manuscript.
ACKNOWLEDGMENTS
This work was financially supported by the Emergency Key Program of Guangzhou Laboratory (Nos. EKPG21-29 and EKPG21-31), the Emergency Grants for SARS-CoV-2 Prevention and Control of Guangdong Province (Nos. 2022A1111090002 and 2021A1111110001), and Key R&D Program of Guangdong Province (No. 2021A1111110002).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The raw RNA-sequencing data reported in this paper have been deposited in the Genome Sequence Archive for Humans under accession code HRA002352 and are accessible at https://ngdc.cncb.ac.cn/search/?dbId=hra&q=HRA002352.




