GII.17[P17] and GII.8[P8] noroviruses showed different RdRp activities associated with their epidemic characteristics
Junshan Gao and Zilei Zhang contributed equally to this work.
Abstract
Norovirus is the primary foodborne pathogenic agent causing viral acute gastroenteritis. It possesses broad genetic diversity and the prevalence of different genotypes varies substantially. However, the differences in RNA-dependent RNA polymerase (RdRp) activity among different genotypes of noroviruses remain unclear. In this study, the molecular mechanism of RdRp activity difference between the epidemic strain GII.17[P17] and the non-epidemic strain GII.8[P8] was characterized. By evaluating the evolutionary history of RdRp sequences with Markov Chain Monte Carlo method, the evolution rate of GII.17[P17] variants was higher than that of GII.8[P8] variants (1.22 × 10−3 nucleotide substitutions/site/year to 9.31 × 10−4 nucleotide substitutions/site/year, respectively). The enzyme catalytic reaction demonstrated that the Vmax value of GII.17[P17] RdRp was 2.5 times than that of GII.8[P8] RdRp. And the Km of GII.17[P17] and GII.8[P8] RdRp were 0.01 and 0.15 mmol/L, respectively. Then, GII.8[P8] RdRp fragment mutants (A−F) were designed, among which GII.8[P8]-A/B containing the conserved motif G/F were found to have significant effects on improving RdRp activity. The Km values of GII.8[P8]-A/B reached 0.07 and 0.06 mmol/L, respectively. And their Vmax values were 1.34 times than that of GII.8[P8] RdRp. In summary, our results suggested that RdRp activities were correlated with their epidemic characteristics. These findings will ultimately provide a better understanding in replication mechanism of noroviruses and development of antiviral drugs.
1 INTRODUCTION
Norovirus (NoV) is a main foodborne pathogen of acute gastroenteritis. It possesses high genetic and antigenic diversity, which is mainly caused by the accumulation of point mutations during genome replication and recombination between variant strains. Among which, recombination may lead to the prevalence of new variants.1-3 NoV has been divided into 10 different genogroups (GI-GX),4 in which GI, GII, GIV, GVIII, and GIX are capable of infecting humans. According to the amino acid sequences of major capsid protein and RNA-dependent RNA polymerase (RdRp) sequence, NoV can be divided into 48 genotypes and 60 P-types.4
Previous researchers found that the obtaining of the RdRp gene with a higher mutation rate through recombination increased the viral genetic diversity and environmental adaptability under selective pressure.2, 5, 6 The mutation rate during RdRp replication was found to be one of the important factors affecting the rapid evolution of NoVs.7 Low conservation of NoVs RdRp played an important role in the viral spread, epidemic, and pathogenicity, which also led to environmental adaption and incessant evolution.8
High mutation rate is one of the distinctive features of RNA viruses,9 providing an evolutionary mechanism for increased survival and transmission. The structure of NoVs RdRp resembles a partially closed right-hand, including finger, thumb, and palm domains like other positive-strand RNA viruses.10 Finger and thumb domains form a closed channel. And the inner of the channel contains highly conserved amino acid motifs A−G.
The prevalence of different NoVs genotypes vary substantially. GII.4 NoV has always been the predominant genotype that has caused six global pandemics since the mid-1990s.11-13 A novel GII.17 Kawasaki 2014 variant emerged in East Asia in the winter of 2014−2015, and was subsequently reported globally.14, 15 It was the first time that a non-GII.4 genotype surpassed the GII.4 NoV as the predominant variant.16, 17 However, most of the other NoV genotypes have not been reported to be epidemics. Among them, GII.8 is the representative genotype with the low detection rate,18 of which only five genome sequences have been reported so far. Furthermore, epidemic genotypes of NoVs in Guangzhou have been demonstrated in our previous surveillance,19, 20 and capsid proteins have been confirmed to be the major factor in the epidemiological characteristics.21, 22 Furthermore, RNA polymerase plays an important role in viral transmission and epidemics.7, 8 However, viral prevalence associated with RdRp genes of different NoV genotypes remains unclear. To unveil the replication mechanism of NoVs correlated with the RdRp region, the epidemic strain GII.17[P17] and the non-epidemic strain GII.8[P8] were systematically compared for evolutionary rates and RdRp replication properties in this study.
2 MATERIALS AND METHODS
2.1 Evolutionary rates of NoVs RdRp genes
The evolutionary rates of GII.8[P8] RdRp and GII.17[P17] RdRp genes were estimated by BEAST v1.7.4 software package.23 The best model suggested by the Bayesian Information Criterion was HKY (Hasegawa, Kishino, and Yano) +I+G model in jModelTest 2.1.6,24 and the codon-based Shapiro−Rambaut−Drummond−2006 (SRD06) nucleotide substitution model was finally chosen because it assumed the HKY substitution model and effectively captured the substitution patterns of different codon positions.25 The Markov Chain Monte Carlo (MCMC) chain lengths were 30 000 000 generations with sampling every 10 000 generations and removed 10% as burn-in. The effective sample size (ESS) was assessed by Tracer v1.6 and all chains were run sufficiently to achieve convergence (ESS above 200). Statistical uncertainty in parameter values was reflected in the 95% highest posterior density (HPD) intervals.
2.2 NoV strains
NoV strains GII.8[P8]-L601 (GenBank accession number MK213549) and GII.17[P17]-L343 (GenBank accession number KT970376) were collected from patients with acute gastroenteritis in our previous study.18, 26, 27
2.3 Expression and purification of recombinant RdRp proteins
NoV positive stool samples were diluted to a 10% suspension with diethylpyrocarbonate-treated water, total viral RNA was purified from the fecal suspension using the HiPure viral RNA kit (Magen). The NoV RdRp genes were amplified via one-step reverse transcription PCR (TAKARA) with primers listed in Table 1. The RdRp sequences were cloned into pGEX-4T-1 vectors. After sequence confirmation by sequencing, the constructs were expressed in Escherichia coli strain BL21 at room temperature overnight and induced with 0.5 mM isopropyl-β-d-thiogalactopyranoside. The RdRp-GST fusion proteins were purified by HiTrap affinity columns GSTrap HP in AKTA Avant system (GE Healthcare). Thrombin (10 units) (GE Healthcare) was used to release the RdRp protein from the GST tag. Purified RdRp proteins were further verified by sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
| Primer | Sequence (5′-3′)a | Orientation |
|---|---|---|
| GII.8[P8] Forward | CGGAATTCGGGGGAGAGGACCACG | Sense |
| GII.8[P8] Reverse | ATTTGCGGCCGCTTCGACGCCATCTTCA | Antisense |
| GII.17[P17] Forward | CGGGATCCGGAGGTGACAACAAGG | Sense |
| GII.17[P17] Reverse | ATTTGCGGCCGCTTCGACGCCATCTTCA | Antisense |
- Abbreviation: RdRp, RNA-dependent RNA polymerase.
- a Sequences in italic are restriction enzyme sites.
2.4 Design of GII.8[P8] RdRp mutants
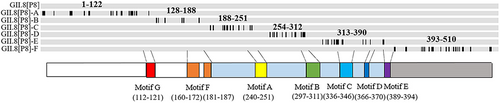
The amino acid sequences of GII.17[P17] RdRp and GII.8[P8] RdRp were aligned by MEGA X (https://www.megasoftware.net). Fragment mutants of GII.8[P8] RdRp were rationally designed based on the conserved motifs of NoV RdRps and alignment results.10 Six amino acid fragments of GII.8[P8] RdRp region were replaced with the fragments corresponding to GII.17[P17] RdRp region, resulting in six fragment mutants of GII.8[P8] RdRp, namely A−F.
2.5 Enzymatic activity of NoV RdRp
A de novo RNA synthesis method was used to evaluate the enzymatic activity of NoV RdRp.1, 28 Fluorescent dye PicoGreen which can specifically bind to dsRNA, was added to the reaction mixture for polymerase activity examination. The standard reaction system was 25 μl with final concentration of 40 µg/ml poly(C) (Sigma-Aldrich), 0.5 mmol/L GTP (Life Technologies), 2.5 mmol/L MnCl2, 5 mmol/L dithiothreitol (DTT), 20 mmol/L Tris-HCl (pH 7.5), and purified NoV RdRp (0, 1, 3, 5 μg). Reaction mixtures were transferred into black 96-well microplates with 175 µl fluorescent dye added to a final volume of 200 µl. Microplates was incubated in the dark for 5 min before measuring fluorescence intensity by microplate reader (excitation and emission wavelengths were 485 and 520 nm, respectively). The relative fluorescence unit (RFU) was the average fluorescence value of the blank control (without RdRp) subtracted from measured fluorescence value.
2.6 Kinetics of NoV RdRp activity
The enzyme kinetics experiment of NoV RdRp is to vary the concentration of substrate (0−0.6 mmol/L) in the standard reaction system and to determine the Km (substrate concentration that yield a half-maximal velocity) and Vmax (maximum velocity). Purified RdRp protein (2 µg) was added to reaction mixtures. Then kinetics of substrate utilization was analyzed by GraphPad Prism 8.0 software with nonlinear regression model, and the Michaelis−Mentent equation to the reaction of RdRp catalyzing GTP was determined: Y = Vmax × X/(Km + X).
3 RESULTS
3.1 Evolutionary rates of NoV RdRp genes
For the lack of enough complete RdRp sequences of GII.8[P8] NoVs (less than 10), partial RdRp sequences (nucleotide position 4358−5102 according to the reference GII.8|MK213549) were also taken into consideration. Thus, 15 strains of GII.8[P8] and 119 strains of GII.17[P17] were chosen to establish the data set for evolutionary rate calculation. The evolutionary rate of GII.17[P17] RdRp was estimated to be 1.22 × 10−3 nucleotide substitutions/site/year (95% HPD interval, 8.54 × 10−4 to 1.57 × 10−3), which was higher than that of GII.8[P8] RdRp (9.31 × 10−4 nucleotide substitutions/site/year, 95% HPD interval, 2.34 × 10−4 to 1.70 × 10−3).
3.2 Expression and verification of NoV RdRp proteins
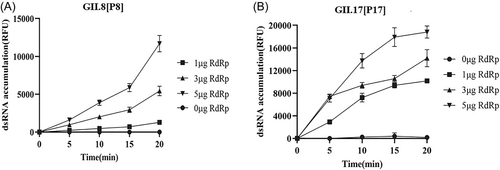
Recombinant NoV RdRp proteins were found to possess high affinity with GST column (Supporting Information: Figure S1A, S1B). Purified RdRp proteins were obtained with molecular mass of approximately 57 kDa, as expected (Supporting Information: Figure S1C, S1D). Enzyme activities of the purified GII.8[P8] RdRp and GII.17[P17] RdRp were evaluated (shown in Figure 1A,B). No obvious fluorescent signal was detected in the negative control. After 20 min reaction, the fluorescence intensity increased to 11 682 ± 1081 and 18 814 ± 1079 RFU when 5 µg RdRp proteins of the two NoV strains were added to the reaction system. It could found that rates of dsRNA synthesis increased with the increasing amount of purified RdRp protein (0−5 µg).
3.3 Substrate kinetics of NoV RdRp
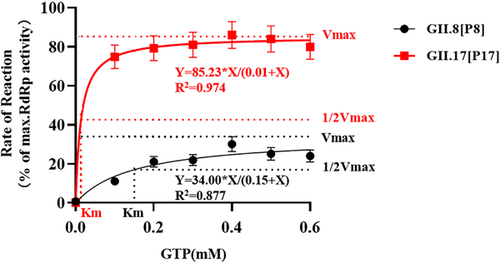
RdRp activities were measured under substrate concentrations ranged from 0 to 0.6 mmol/L, consistent with the Michaelis−Menten model for enzyme kinetics (Figure 2). The RdRp activity of the epidemic strain GII.17[P17] was higher than that of the non-epidemic strain GII.8[P8]. In detail, the Km of GII.17[P17] RdRp was 0.01 mmol/L, while the Km of GII.8[P8] RdRp was 0.15 mmol/L. This indicated that GII.17[P17] RdRp was found to possess higher affinity for the substrate, and the Vmax could be reached under a lower substrate concentration. Besides, the Vmax of GII.17[P17] RdRp was 1.5 times higher than that of GII.8[P8] RdRp.
3.4 Identification of key amino acid fragments affecting NoV RdRp activity
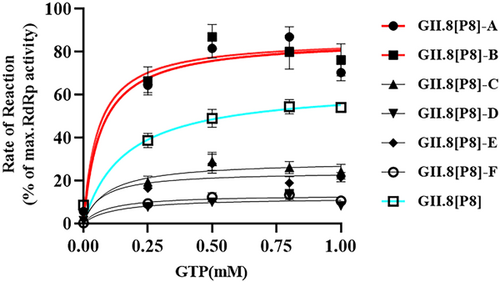
Six fragment mutants (GII.8[P8] A−F) were designed to cover the entire RdRp region (Figure 3). Enzyme activity of each RdRp mutant was evaluated by measuring the rate of enzymatic reaction under different substrate concentrations (Figure 4). Enzyme activities of GII.8[P8] RdRp fragment mutants were compared with the wide-type GII.8[P8] RdRp. Among which, Vmax values of both GII.8[P8]-A and GII.8[P8]-B were 1.34 times higher than that of GII.8[P8] RdRp (Table 2). Moreover, the Km of GII.8[P8]-A and GII.8[P8]-B mutants with the conserved motifs G and F were decreased to 0.07 and 0.06 mmol/L, respectively. Enzyme activities of GII.8[P8] C-F mutants was found to be decreased.
| RdRp | rVmaxa | Km (GTP), Michaelis−Menten model (mmol/L) at 30°C |
|---|---|---|
| GII.8[P8]-A | 1.34 | 0.07 |
| GII.8[P8]-B | 1.34 | 0.06 |
| GII.8[P8]-C | 0.45 | 0.09 |
| GII.8[P8]-D | 0.19 | 0.12 |
| GII.8[P8]-E | 0.38 | 0.07 |
| GII.8[P8]-F | 0.21 | 0.09 |
| GII.8[P8] | 1.00 | 0.16 |
- Abbreviations: NoV, norovirus; RdRp, RNA-dependent RNA polymerase.
- a rVmax represents the relative Vmax, which is the ratio of the Vmax value of the GII.8[P8] RdRp fragment mutant to the Vmax value of GII.8[P8] RdRp.
4 DISCUSSION
NoVs possess broad genetic diversity with 48 genotypes, more than 30 of which can infect humans. Recombination (ORF1/ORF2) and mutation are important mechanisms for the diversity and the emergence of new NoV variants.1, 29, 30 Research on RNA viruses have shown that the epidemiological adaptability of viruses depends on many factors, such as the viral replication rate, genetic diversity, and host.9 Both virus replication rate and genetic diversity are related to the RdRp activity. NoVs RdRp activities of the epidemic strain GII.17[P17] and the non-epidemic strain GII.8[P8] were characterized to explore the related molecular mechanisms in this study.
RdRp is a key enzyme for transcription and replication of NoV genome. Bull et al.31 proposed that the GII.4 capsid sequence had an average of 1.7-fold higher rate of evolution than non-epidemic strains, which may be the interpretation of the high replication rate and the high mutation rate of GII.4. Mahar et al.32 found that the evolution rates of GII.3 strains were 4.16 × 10−3 to 6.97 × 10−3 nucleotide substitutions/site/year from 1975 to 2010, indicating an increasing in genetic diversity and environmental adaptation under selective pressure by recombining RdRp genes with higher mutation rates. The epidemic strain GII.17[P17] and the non-epidemic strain GII.8[P8] were chosen due to their epidemic disparity in this study. The strict clock model indicated that GII.17[P17] RdRp gene and GII.8[P8] RdRp gene evolved at the rate of 1.22 × 10−3 (95% HPDs, 8.54 × 10−4 to 1.57 × 10−3) nucleotide substitutions/site/year and 9.31 × 10−4 nucleotide substitutions/site/year (95% HPDs, 2.34 × 10−4 to 1.70 × 10−3), respectively. Our results demonstrated that epidemic strains would have a faster evolution rate which was consistent with previous reports.31
The functions of RdRp are highly correlated to the epidemic of NoVs. Bull et al.33 found that the kinetic activity of the NoV epidemic strain GII.4 was higher than that of the NoV non-epidemic strain GII.7. In detail, the Km values of GII.4 RdRp and GII.7 RdRp were 0.06 and 0.13 mmol/L, and their Vmax values were 8944 and 14233 pmoles h−1 μg,−1 respectively. In this study, the Km values of GII.17[P17] RdRp and GII.8[P8] RdRp were estimated to be 0.01 and 0.15 mmol/L, respectively. And the Vmax value of GII.17[P17] RdRp was 2.5 times higher than that of GII.8[P8] RdRp. These results suggested that the RdRp activities of different NoV genotypes were interrelated to their epidemic characteristics.
In this study, conserved motifs were designed into fragment mutants, and their enzyme activity were compared based on substrate kinetics analyses. The Km values of GII.8[P8]-A and B mutants containing the conserved motif G/F were reduced to 0.07 and 0.06 mmol/L, the Vmax values of above mutants were both 1.34 times higher than that of GII.8[P8] RdRp. These findings indicated that the amino acid mutation of the RdRp conserved motifs affected the viral replication rate. The mutation of motif F had been confirmed to catalyze the transfer of nucleoside triphosphates and promote the synthesis of RNA,31 which could further have effects on NoV adaptability and epidemic characteristics. Specific amino acid sites of motif G/F would be needed to be further verified on their effects.
5 CONCLUSIONS
In this study, the RdRp activity of the epidemic strain GII.17[P17] and the non-epidemic strain GII.8[P8] were characterized systematically. And the results demonstrated that RdRp evolution rates were consistent with their epidemic characteristics. By designing GII.8[P8] RdRp mutants, key amino acid fragments containing conserved motifs G and F were found to have important effects on the RdRp activity of non-epidemic strains. Our findings could provide a better understanding in molecular mechanisms on NoV epidemicity and antiviral drugs development.
AUTHOR CONTRIBUTIONS
Junshan Gao, Liang Xue, Qingping Wu, Ying Li, Tong Cheng, Luobing Meng, Yijing Li designed and implemented the study. Junshan Gao, Zilei Zhang, Tong Cheng collected and analyzed the data. Junshan Gao and Zilei Zhang drafted the manuscript. All authors discussed the results of the study and agreed to the published version of the manuscript.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (32272436), the Natural Science Foundations of Guangdong Province for Distinguished Young Scholars (2019B151502065), and the Key Research and Development Program of Guangdong Province (2019B020209001).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




