Hydrogen sulfide inhibits human T-cell leukemia virus type-1 (HTLV-1) protein expression via regulation of ATG4B
Abstract
Hydrogen sulfide (H2S) is a redox gasotransmitter. It has been shown that H2S has a key role in host antiviral defense by inhibiting interleukin production and S-sulfhydrating Keap1 lead to Nrf2/ARE pathway activation. However, it is yet unclear whether H2S can play an antiviral role by regulating autophagy. In this study, we found that exogenous H2S decreased the expression of human T-cell leukemia virus type-1 (HTLV-1) protein and HTLV-1 induced autophagosomes accumulation. Transmission electron microscope assays indicated that autophagosomes accumulation decreased after H2S administration. HTLV-1-transformed T-cell lines had a high level of CSE (H2S endogenous enzyme) which could be induced in Hela by HTLV-1 infection. Immunoblot demonstrated that overexpression of CSE inhibited HTLV-1 protein expression and autophagy. And we got the opposite after CSE knockdown. Meanwhile, H2S could not restrain the autophagy when ATG4B had a mutant at its site of 89. In a word, these results suggested that H2S modulated HTLV-1 protein expression via ATG4B. Therefore, our findings suggested a new mechanism by which H2S defended against virus infection.
Abbreviations
-
- 3-MA
-
- 3-methyladenine
-
- ARE
-
- antioxidant response element
-
- ATL
-
- adult T-cell leukemia
-
- CBS
-
- cystathionine β-synthase
-
- CO
-
- carbon monoxide
-
- CSE
-
- cystathionine γ-lyase
-
- H2S
-
- hydrogen sulfide
-
- HTLV-1
-
- human T-cell leukemia virus type-1
-
- MST
-
- 3-mercaptopyruvate sulfur transferase
-
- NO
-
- nitric oxide
-
- RA
-
- rapamycin
1 BACKGROUND
H2S (hydrogen sulfide) is a colorless gas with lipid soluble. It has a smell of rotten eggs and has been supposed to be a kind of poisonous gas previously. Lately, it has been reported that H2S could be produced in mammalian cells.1-4 There are different concentration of H2S in different part. For example, it is about 46 μmol L−1 in rat serum and 100 μmol L−1 in rat brain.5, 6 Endogenous H2S is produced in the cells by three enzymes, cystathionine γ-lyase (CSE), cystathionine β-synthase (CBS), and 3-mercaptopyruvate sulfur transferase (MST).7-9 CBS is the main enzyme in the central nervous system,6, 10, 11 CSE is the key enzyme in the cardiovascular system,12, 13 whereas MST is responsible for the production of H2S in the kidney.14, 15 In addition, H2S has a pivotal role in digestive system and respiratory system.16-19 Consequently, H2S is considered as the third gasotransmitter, such as carbon monoxide (CO) and nitric oxide (NO).20-22
Sulfhydration is the main mechanism of H2S in regulating protein activity.23, 24 In this modification, H2S changes a Cys residue (–SH) of a target protein into −SSH.23, 24 For example, H2S can restain Keap1 (Kelch-like ECH-associated protein-1) activity by sulfhydration of Cys 151, and then Nrf2 translocates into the nucleus and combine with antioxidant response element (ARE).25, 26 This results in the expression of antioxidant genes.
Autophagy is a degradation process that transmits cytoplasmic components to lysosomes via autophagosomes.27, 28 Lipidation of LC3 is important for early stage of autophagy membrane formation.29, 30 ATG4, a kind of cysteine protease, perform as executer to process LC3 lipidation and delipidation of lipidated LC3.31, 32 There are four ATG4 isoforms in mammal cells, such as ATG4A, ATG4B, ATG4C, and ATG4D.33 ATG4B is the key isoform for cleaving LC3/GABARAP.34 ATG4A has the ability of processing GABARAP. Compared with ATG4B, the activity of ATG4A is weak.35 However, ATG4D and ATG4C have almost no activity in vitro.34, 36
Adult T-cell leukemia (ATL), a hematologic malignant disease of adult, is directly related to the infection of human T-cell leukemia virus type 1 (HTLV-1).37-39 It has a variety of symptoms like acute type, chronic type, or insidious type.40, 41 Chronic and insidious patients are often supported by symptomatic support treatment; Chemical and biological therapy are often used to cure acute lymphoma. However, the effect is unsatisfactory, and the median survival of the acute patients is about 2–6 months.40, 42-44
Tax, HTLV-1 protein, can continuously activate autophagy and generate a lot of autophagosomes. And then, HTLV-1 is wrapped in the autophagosomes. HTLV-1 in autophagosomes not only can get away from intracellular immunity but also complete self-replication in autophagosomes.45, 46 The massive virus promoted the expression of Tax. Lots of Tax continue to activate autophagy and advance virus replication via positive feedback, resulting in the generation and development of ATL finally.47
In this study, we demonstrated that CSE was induced by HTLV-1 infection and suppressed HTLV-1 protein expression. We showed that exogenous H2S inhibited the accumulation of autophagosomes during HTLV-1 infection. Furthermore, CSE and exogenous H2S were associated with ATG4B which is essential for autophagy early period. Collectively, our findings may shed some new lights on HTLV-1 therapy and contribute to our understanding of H2S defenses against virus infection.
2 METHODS
2.1 Regents
The following antibodies were used for immunoblot analysis: anti-Gapdh (60004-1-Ig; Proteintech), anti-CBS (14782; Cell Signaling Technology), anti-CSE (19689; Cell Signaling Technology), anti-HTLV-1 p19 (ab9080; Abcam), anti-MST (ab224043; Abcam), anti-Tax (sc-57872; Santa Cruz Biotechnology), anti-ATG4B (15131-1-AP; Proteintech), anti-LC3A/B (18725-1-AP; Proteintech). The 3-MA (M9281), NaHS (151627), and RA (553211) were obtained from Sigma-Aldrich. SYBR Green quantitative PCR master mix were purchased from TOYOBO Ideas and Chemistry (QPK-201). MTT Cell Proliferation and Cytotoxicity Assay Kit was obtained from Beyotime Biotechnology.
2.2 ATG4B mutants' preparation
ATG4B plasmid was bought from Sino Biological (Cat. HG20407-ANG). GeneArt® Site-Directed Mutagenesis PLUS Kit was used to construct cysteine mutants. Gene-specific primer sequences were as follows:
C78A:
Forward: CACAGGCTGGGGCTGCATGCTGCGGGCTGGACAGATGATCTTTGCCCAAGCC
Reverse: GGCTTGGGCAAAGATCATCTGTCCAGCCCGCAGCATGCAGCCCCAGCCTGTG
C89A:
Forward: GATGATCTTTGCCCAAGCCCTGGTGGCCCGGCACCTAGGCCGAGATTGGAGG
Reverse: CCTCCAATCTCGGCCTAGGTGCCGGGCCACCAGGGCTTGGGCAAAGATCATC
C183A:
Forward: GTTGTGATGGAGGAAATCAGAAGGTTGCCAGGACCAGCGTTCCCTGTGCAGGC
Reverse: GCCTGCACAGGGAACGCTGGTCCTGGCAACCTTCTGATTTCCTCCATCACAAC
C189A:
Forward: GAAGGTTGTGCAGGACCAGCGTTCCCGCTGCAGGCGCCACTGCGTTTCCTGCAG
Reverse: CTGCAGGAAACGCAGTGGCGCCTGCAGCGGGAACGCTGGTCCTGCACAACCTTC
C203A:
Forward: GTTTCCTGCAGATTCCGACCGGCACGCCAACGGATTCCCTGCCGGAGCTGAG
Reverse: CTCAGCTCCGGCAGGGAATCCGTTGGCGTGCCGGTCGGAATCTGCAGGAAAC
C246A:
Forward: GGCCTACGTGGAGACGCTGAAGCACGCCTTCATGATGCCCCAGTCCCTGGGC
Reverse: GCCCAGGGACTGGGGCATCATGAAGGCGTGCTTCAGCGTCTCCACGTAGGCC
C292A:
Forward: GCCAGCCGTGGAGCCCACTGATGGCGCCTTCATCCCGGACGAGAGCTTCCAC
Reverse: GTGGAAGCTCTCGTCCGGGATGAAGGCGCCATCAGTGGGCTCCACGGCTGGC
C301A:
Forward: CTTCATCCCGGACGAGAGCTTCCACGCCCAGCACCCGCCGTGCCGCATGAGC
Reverse: GCTCATGCGGCACGGCGGGTGCTGGGCGTGGAAGCTCTCGTCCGGGATGAAG
C323A:
Forward: CCCGTCCATCGCTGTGGGGTTTTTCGCTAAGACTGAAGATGACTTCAATGATTG
Reverse: CAATCATTGAAGTCATCTTCAGTCTTAGCGAAAAACCCCACAGCGATGGACGGG
C333A:
Forward: GACTGAAGATGACTTCAATGATTGGGCCCAGCAAGTCAAAAAGCTGTCTCTG
Reverse: CAGAGACAGCTTTTTGACTTGCTGGGCCCAATCATTGAAGTCATCTTCAGTC
C361A:
Forward: GGAGCTGCAGCCTTCACATCTGGCCGCCCCCGACGTCCTGAACCTGTCCCTAG
Reverse: CTAGGGACAGGTTCAGGACGTCGGGGGCGGCCAGATGTGAAGGCTGCAGCTCC
2.3 Cell culture and transfection
Hela cells were cultured in Dulbecco's modified Eagle's medium with high glucose. MT2, MT4, C8166, and Jurkat cells were grown in RPMI-1640. All cells were provided with 10% fetal bovine serum (FBS) (Gibco), 4 mM l-glutamine under humidified conditions with 5% CO2 at 37°C. Transfection of Hela cells was performed with Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions.
2.4 Western blot
Immunoblot analysis was performed. Hela cells were transfected with various combinations of plasmids or small interfering RNA (siRNA). At 24−36 h after the transfection, the cell lysates were prepared in RIPA lysis buffer plus a protease inhibitor “cocktail” (Roche). MT2, MT4, C8166, and Jurkat cells were simulated by NaHS (100 µmol/L) for 2 or 3 days. The cell lysates were incubated with indicated antibodies and analyzed by immunoblot.
2.5 Real-time polymerase chain reaction (PCR)
Total RNA was extracted from the cultured cells with TRIzol reagent (Invitrogen) as described by the manufacturer. All gene transcripts were quantified by real-time PCR with the ABI 7500 Fast real-time PCR system (Applied Biosystems). The results were analyzed using the method. The primers used for real-time PCR were as follows:
Tax,
Forward: 5′-CGGATACCCAGTCTACGTGT-3′,
Reverse: 5′-GAGCCGATAACGCGTCCATCG-3′;
GAPDH,
Forward: 5′-GCCAAAAGGGTCATCATCTC-3′,
Reverse: 5′-GTAGAGGCAGGGATGATGTTC-3′.
2.6 Transmission electron microscope
After indicated stimulation, MT2 cells were fixed with the transmission electron microscopy (TEM) fixative. And then let the precipitation resuspended in the fixative and fixed at 4°C for preservation and transportation. The operation was performed according to the instructions. The cuprum grids are observed under TEM (HITACHI: HT7800/HT7700) and take images.
2.7 RNA interference
The siRNA, CSE-silencer, was obtained from Genepharma. The sequences used were as follows: K1, forward, 5′-GCCGAGAGCUUGGGAGGAUTT-3′, reverse, 5′-AUCCUCCCAAGCUCUCGGCTT-3′; K2, forward, 5′-GCAGCCACUGUAACUAUUATT-3′, reverse, 5′-UAAUAGUUACAGUGGCUGCTT-3′. The negative control was purchased from Genepharma (catalog no. A06001).
2.8 Coculture
Hela cells were cultured for 24 h. MT2 cells were seeded and cocultured with Hela cells at a ratio of 1:100 (1 × 104 HTLV-1 donor cells: 1 × 106 uninfected recipient cells ratio) and allowed to incubate for an additional 24 h before harvesting for downstream assays.
2.9 Statistical analysis
The data are presented as the mean ± standard error from at least three independent experiments. All p-values were calculated using two-tailed Student's t tests (Graphpad) and were considered statistically no significant when p > 0.05.
3 RESULTS
3.1 H2S treatment suppressed HTLV1 replication, reduced autophagy level, and had no effect on cells' viability
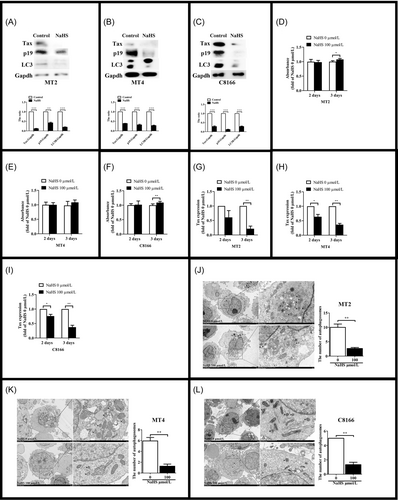
The increase of autophagy is a common manifestation of viral infection. HTLV-1 induces autophagy and completes their replication in autophagosomes. H2S, an endogenous gas signal molecule, can inhibit autophagy.48-50 That's the reason we explore the effect of H2S on HTLV-1 infection. HTLV-1 positive cells, MT2, MT4, and C8166, have high level of autophagy. LC3 has two isoforms, LC3A (upper line) and LC3B (below line). LC3B is a marker of autophagy.51 The expression of LC3B in MT2 reduced after H2S administration for 48 h (Figure 1A), and virus protein Tax and p19 also decreased. We saw the same results in MT4 (Figure 1B) and C8166 (Figure 1C). To determine whether H2S had an effect on cell activity, MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay was performed. We found that H2S had no effect on cell viability after NaHS administration for 2 days, and the cells viability increased after H2S administration for 3 days (Figure 1D–F). Meanwhile, the level of Tax gene expression was inhibited by H2S significantly (Figure 1G–I). Consistently, the accumulation of autophagosomes was decreased in H2S-treated cells compared to the cells without H2S administration (Figure 1J–L). These results suggested that H2S inhibited the expression of HTLV-1 proteins, reduced the level of autophagy. And the effect of H2S was not associated with the decrease of cell viability.
3.2 The inhibition of virus replication by H2S was related with autophagy
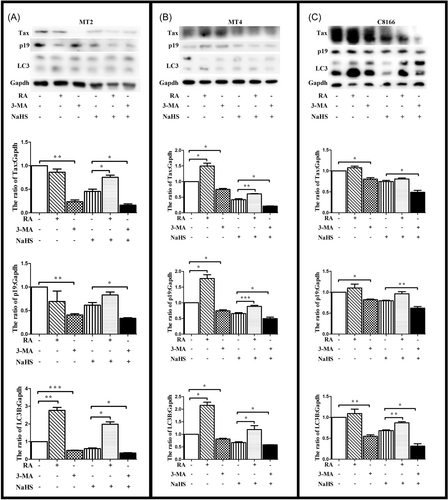
We determined the role of H2S in MT2 cells in the presence of RA (rapamycin) or 3-MA (3-methyladenine), which were believed to activate or block the autophagy. We found that RA could reduce the effect of H2S, and 3-MA could enhance the effect of H2S (Figure 2A). Similar results were observed in MT4 and C8166 (Figure 2B,C). It meant that H2S depressed the expression HTLV-1 related protein via autophagy.
3.3 H2S interacted with ATG4B
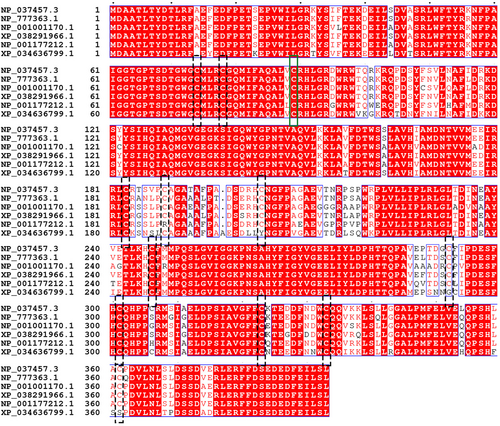
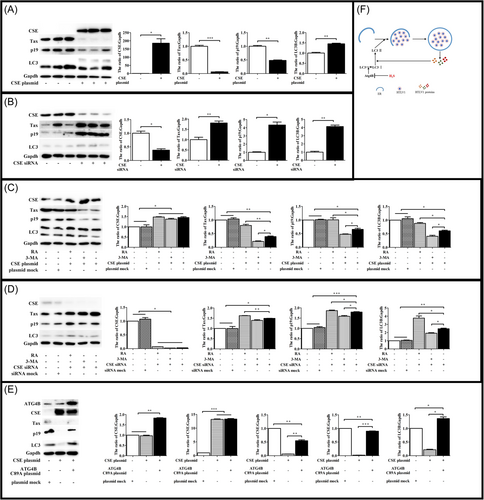
To characterize the mechanism by which H2S regulated autophagosome accumulation, we tested whether H2S was able to interact with ATG4B involved in canonical autophagy. ATG4B is a conservative cysteine proteinase among species,52 which activity dependents on the cysteine located in the active center.53 ATG4B has 12 cysteines according to NCBI database (Figure 3). It has been reported that ATG4B has no activity if the cysteine at site 74 is mutated.54, 55 Meanwhile, H2S signals is by modification of cysteine residues on target proteins in a process designated as sulfhydration or persulfidation. Therefore, we constructed 11 mutants which had one cysteine mutated separately except the site 74.
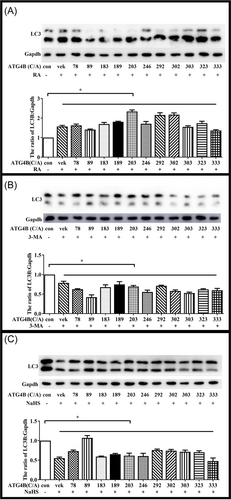
The plasmid of ATG4B and its cysteine mutants were transfected in Hela cells. To confirm the effect of these mutants on ATG4B activity, Hela cells were treated by RA or 3-MA and then subjected to immunoblot analysis. The results showed that RA and 3-MA could active or depress autophagy when ATG4B had these cysteine mutated (Figure 4A,B). It suggested that these cysteine were not essential for ATG4B activity. However, H2S had lose its effect on autophagy when the cysteine at site 89 was mutated (Figure 4C). These data indicated that cysteine 89 of ATG4B was the target of H2S in the regulation of autophagy.
3.4 CSE expression increased in early period of HTLV-1 infection
Then we tried to explore the functions of H2S endogenous enzyme during HTLV-1 infection. Jurkat and MT2 cells were lysated, and H2S endogenous enzymes were investigated. Immunoblot assays demonstrated that the expression of H2S endogenous enzymes were higher in MT2 than Jurkat (Figure 5A). We wondered whether H2S endogenous enzymes would increase in the early period of HTLV-1 infection. Hela cells were cocultured with MT2 cells and the expression levels of Tax was investigated. PCR assays demonstrated that Tax could be detected in Hela cells after cocultured for 24 h (Figure 5B). Then we detected H2S endogenous enzymes in the early period of HTLV-1 infection. We found that the expression of CSE reached a maximum on the 48 h and the level of autophagy decreased after coculture (Figure 5C). However, the expression of CBS and MST decreased after coculture for 48 h (Figure 5C). These results suggested that CSE played a key role in early period of HTLV-1 infection. To confirm that the decrease of autophagy was associated with CSE and HTLV-1. Hela cells were cultured without FBS. Immunoblot assays found that the increase of CSE could not reduce the level of autophagy without HTLV-1 infection (Figure 5D). And then, we detected the effect of exogenous H2S on Hela cells. The result showed that exogenous H2S had no effect on the expression of endogenous enzymes (Figure 5E). In summary, the expression of CSE decreased the level of autophagy of HTLV-1 positive cells.
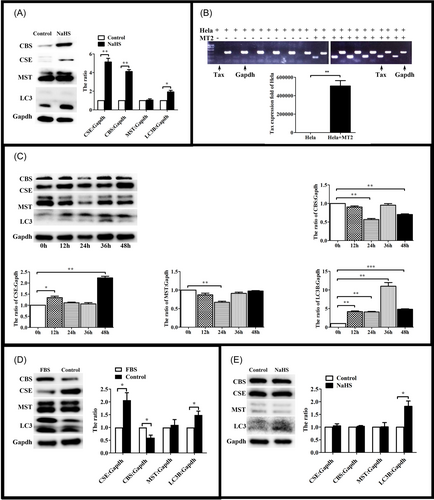
3.5 CSE could inhibit HTLV-1 replication via ATG4B
Next, we wanted to make sure whether CSE had the same effect like exogenous H2S. Hela cells with overexpressing or low expressing of CSE were cocultured with MT2 cells and the expression levels of HTLV-1 proteins and autophagy were investigated. Immunoblot assays demonstrated that the expression of CSE was associated with lower levels of HTLV-1 proteins and autophagy (Figure 6A,B). Meanwhile, we investigated whether RA and 3-MA could impact the effect of CSE. We found that RA could reduce the effect of CSE, and 3-MA could enhanced the effect of CSE (Figure 6C,D). To confirm that ATG4B was associated with the effect of CSE. Hela cells, overexpressing of CSE and ATG4B mutant, were cocultured with MT2 cells. Immunoblot assays showed that the level of HTLV-1 protein was restored partly (Figure 6E). Taken together, these data suggested that the expression of CSE decreased HTLV-1 protein via the cysteine 89 of ATG4B.
4 DISCUSSION
As a gasotransmitter, H2S plays an important role in the physiological and pathological process of the organism.16-19 Nowadays, the mechanism of H2S in the process of virus infection is unclear. It has been reported that H2S could play an antiviral role through its antioxidant and anti-inflammatory effects, such as COVID-9, HSV, HIV, and paramyxovirus.56-61 Our study found that both endogenous and exogenous H2S could inhibit autophagy caused by HTLV-1 and reduced the expression of viral proteins via autophagy-related protein ATG4B.
Our results showed that HTLV-1-positive T-cell lines had a strong expression of CSE and CSE expression could be induced in Hela after HTLV-1 infection. Further studies indicated that the overexpression of CSE inhibited HTLV-1 protein expression and CSE knockdown was associated with higher levels of HTLV-1 protein expression. There was significant relevance between autophagy level and the expression of CSE. These results drove us to find the possible mechanism to explain the role of CSE in autophagy.
There is a complex relationships between autophagy and pathogenic microorganisms.62 Paradoxically, autophagosomes are benefit for HTLV-1 to complete its replication.45-47 We used LC3B as the marker of autophagy and examined the effect of CSE on HTLV-1-induced autophagy. Our findings confirmed the hypothesis by three steps. First, H2S inhibited the autophagosomes accumulation in MT2 cells. Second, CSE inhibited the autophagosomes accumulation induced by HTLV-1 infection in Hela. Third, when RA was used to activate the early stage of autophagy, we found that CSE overexpression lost the abilities to inhibit autophagosomes accumulation and HTLV-1 viral protein expression increased. These results suggested that the regulatory role of CSE in autophagosomes accumulation was important to its effect on HTLV-1 protein expression.
How could H2S regulate autophagosomes accumulation? It has been reported that ATG4B has an important role in LC3B and autophagosomes formation.34 As a cysteine enzyme, ATG4B is a good target for H2S regulation. Our findings indicated that H2S interacted with ATG4B. Using a serious of ATG4B cysteine mutants, we found ATG4B cysteine 89 was essential for the effect of H2S. However, ATG4B will almost lost the ability when it has a mutant at the site of cysteine 74.63 Therefore, we could not confirm whether the cysteine 74 was associated with H2S function.
It is reported that the function of ATG4B can be changed via posttranslational modification. For example, its activity is enhanced when the serine at 383 site was phosphorylated64; After phosphorylation of serine at 34 and 316 sites or S-nitrosation of cysteine at 189 and 292 sites, the opposite results will appear65-67; In addition, when the level of reactive oxygen species increase, a disulfide bond will be formed between cysteines at site 292 and 361 resulting the level of autophagy increases.68 Our study suggested that H2S could sulfurize cysteine at site 89 and inhibit autophagy. However, we had no direct evidence to confirm the sulfhydration of ATG4B because of technical limitations.
5 CONCLUSION
Together, our study demonstrated that H2S modulated autophagosomes accumulation by regulating ATG4B, thereby affected the expression of HTLV-1 proteins. Our research may expand our understanding of H2S on virus infection and suggest a new role of ATGs sulfhydration in autophagy.
AUTHOR CONTRIBUTIONS
Huandi Liu and Hui Wang conceived the idea for the project. Jiaxiang Sun, Shuaifeng Guo, Xuhong Cheng, Zhongxin Zhang, Jia Wan, Chunduo Wang, Xiaoying Zhi, Linghui Yuan did the experiments. Huandi Liu designed experiments, analyzed the results, and wrote the paper.
ACKNOWLEDGMENTS
Dr. Liangwei Duan designed the primers of ATG4B mutants. MT4 and C8166 were gifts from Professor Xiaofei Zhu. We are grateful to Dr. Liangwei Duan and Professor Xiaofei Zhu very much. This study was supported by grants from Key scientific research projects in Universities of Henan Province (20A320011), the National Natural Science Foundation of China (32000612) and 111 Project (D20036).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data underlying the graphs have been presented in the main figures. The materials relating to figures are available from Huandi Liu upon reasonable request.




