CRISPR/Cas9-HPV-liposome enhances antitumor immunity and treatment of HPV infection-associated cervical cancer
Abstract
Increasing evidence shows that human papillomavirus (HPV) E6/E7 deletion in cervical cancer cells may be related to the immunosuppressive tumor microenvironment and adverse reactions or resistance to immune checkpoint blockade. Here, we demonstrate that liposome delivery of CRISPR/cas9 can effectively knock out HPV, which, in turn, induces autophagy and triggers cell death-related immune activation by releasing damage-related molecular patterns. The results of in vivo experiments showed that HPV-targeting guide RNA–liposomes could promote CD8+ T cell infiltration in tumor tissues; enhance the expression of proinflammatory cytokines, such as interleukin-12, tumor necrosis factor-α, and interferon-γ, and reduce regulatory T cells and myeloid suppressor cells. The combination of HPV-targeting guide RNA–liposomes with immune checkpoint inhibitors and antiprogrammed death-1 antibodies produced highly effective antitumor effects. In addition, combination therapy induced immune memory in the cervical cancer model.
1 INTRODUCTION
Evidence suggests that only less than 30% of patients with cervical cancer benefit from programmed cell death-1 (PD-1)/programmed cell death ligand-1 (PD-L1) inhibitors, and patients with other cancer types have poor response or resistance to immune checkpoint blockade (ICB).1, 2 Our previous studies have shown that human papillomavirus (HPV) is directly involved in the regulation of antitumor immunity.3 For example, HPV infection is remarkably associated with reduced T cell infiltration at the tumor site and adverse reactions or drug resistance to PD-1 blocking therapy.4 HPV also contributes to the accumulation of suppressive immune cells, such as bone marrow-derived suppressor cells (MDSCs) and regulatory T (Treg) cells, and forms an immunosuppressive tumor microenvironment (TME) during tumourigenesis and development.5 In addition, HPV knockout can induce autophagy,6-8 and the downregulation of HPV function can effectively support the occurrence of cervical cancer.
Recently, CRISPR/cas9 has been developed as a promising new tool to inhibit gene expression. We successfully developed a liposome platform for the systemic administration of CRISPR/cas9 in cervical cancer.9 HPV gene knockout mediated by guide RNA (gRNA)–liposome can remarkably inhibit the growth of human cervical cancer cells in vitro and in vivo.10 We developed a liposome platform for delivering CRISPR/cas9 to cervical cancer cells to explore whether HPV knockout-induced tumor cell death is accompanied by the release of the damage-related molecular pattern (DAMP) that can trigger antitumor immune activation. Our results showed that these HPV-targeting gRNA–liposomes can restore the sensitivity of tumor cells to death, release DAMP, activate autophagy, and promote the secretion of additional DAMP and autophagosomes. In vivo experimental results showed that HPV repair induced a strong CD8+ T cell response and reversed the immunosuppressive TME by reducing the expression of Treg and monocyte MDSCs (Mo-MDSCs) and increasing the expression of proinflammatory cytokines. In addition, we also evaluated the antitumor effect of CRISPR combined with anti-PD-1 immunotherapy in a cervical tumor model and proved the remarkable therapeutic effect and immune memory of this combined strategy. Our results suggested that the tumor inhibitory repair of CRISPR nanodrugs may improve the sensitivity of ICB and provide an effective combination treatment for a variety of malignant tumors.
2 MATERIALS AND METHODS
2.1 Study design
gRNA (HPV16 E6/E7) expression plasmids were constructed according to the manufacturer's protocol.9 Briefly, to prepare a 100-bp double-stranded DNA(dsDNA) insert fragment containing the target sequence (20 bp) and a protospacer-adjacent motif sequence, we used a set of oligonucleotides and generated the fragment using T4 PNK (NEB). The dsDNA fragments were purified and inserted into the BbsI site of a gRNA cloning vector with T4 DNA ligase (NEB). Detailed BLAST searching of human and murine genomes was carried out to identify potential off-target binding of HPV gRNAs. Two sets of oligonucleotides were designed. All oligonucleotides were synthesized and purified by Sangon Biotech Co. The single-guide RNA sequence (CACCGCAACAGTTACTGCGACGTG, AAACCACGTCGCAG TAACTGT TGC)-targeting E6 and (CACCGACACGTAGACATTCGTACTT, AAACAAGTACGAAT GTCTA CGTGT)-targeting E7.
A liposome was developed to deliver HPV gRNA, and then its transfection efficiency and antitumor effect on SiHa cancer cells were evaluated by activity assay, real-time polymerase chain reaction (RT-PCR), immunofluorescence, and confocal imaging. For the mechanism study, immunogenic cell death (ICD) and tumor cell autophagy induced in vitro (n = 3 replicates in each group) and changes in immune cell number and phenotype in vivo (n = 5 mice in each group) were analyzed by flow cytometry, immunofluorescence imaging, and enzyme-linked immunosorbent assay (ELISA). A subcutaneous mouse model was established to analyze the antitumor efficacy of HPV gRNA–liposomes alone, anti-PD-1 alone, and their combination (five mice in each group) and evaluate the effect of HPV knockout on ICB. The animals were randomly assigned to a non-blinded treatment group.
2.2 Preparation of blank nanoliposomes
Blank nanoliposomes were prepared using the thin film method based on our previous study.10
2.3 Preparation of nanoliposome–CRISPR/Cas9 complex
The self-assembly method was used as previously described10 to prepare the liposome–CRISPR/Cas9 complex.
2.4 In vitro cytotoxicity and apoptosis evaluation
Cancer cells were seeded in 96-well plates at a density of 3–5 × 104 cells per well and incubated with 100 μl of medium containing 10% fetal bovine serum (FBS; Gibco) for 15 h. Then, the old medium was removed, and 100 μl of fresh medium containing phosphate-buffered saline (PBS), naked HPV gRNA, liposome, and HPV gRNA–liposome with different concentrations was added and cultured for 48 h. Afterward, the plates were cleaned with PBS and added with a fresh medium containing 10% (vol/vol) alamarBlue (Thermo Fisher Scientific). After 2 h of incubation, the cells were measured with a microplate reader (Infinite M200 Pro; Tecan). The UV–vis absorptivity (a) of all samples was measured at 450 and 690 nm (background). Cell survival rate (%) was calculated using the formula: (asample ablank)/(acontrol ablank) × 100.
2.5 Establishment and identification of the humanized-peripheral blood leukocyte-severe combined immune deficiency mice model
Severe combined immunodeficiency (SCID) mice weighing 18–20 g and aged 4–5 weeks were purchased from the Experimental Animal Center of the Academy of Military Medical Sciences. The mice were individually caged in specific pathogen-free cages. All animal experiments and programs were conducted in strict accordance with the Guidelines for the Care and Use of Laboratory Animals issued by the National Institutes of Health. The animal experiments were approved by the Institutional Animal Welfare Committee. Humanized-peripheral blood leukocyte (hu-PBL)-SCID mice were established as previously described.
2.6 Antitumor immune responses induced by HPV gRNA–liposome
A siHa-bearing tumor mouse model was prepared as follows. About 4 × 105 cells in 100 μl of PBS were implanted subcutaneously on the right flank of 6-week-old female hu-PBL-SCID mice. The mice were randomly divided into three groups (n = 5 mice per group), which received 200 μl of PBS, liposome, or HPV gRNA–liposome via tail vein on Days 7, 10, and 13 after tumor implantation. On Day 15 (2 days after the last injection), the mice were euthanized and the tumors and groin lymph nodes were isolated to examine the number and phenotype of immune cells, such as T cells, dendritic cells (DCs), and MDSCs, and the concentrations of secreted cytokines.
2.7 ATP, high-mobility group box protein-1, and cytokine detection
Next, the release of ATP and high-mobility group box protein-1 (HMGB1) by tumor cells was evaluated. The cells were seeded in a six-well plate at a density of 5 × 105 cells/well and incubated with 1.5 ml of medium containing 10% FBS for 15 h. The medium was removed, and 1.5 ml of fresh medium containing PBS, naked HPV gRNA, liposome, or HPV gRNA–liposome was added and cultured for 48 h. The cell culture medium was collected and measured using ATP (Invitrogen) and HMGB1 ELISA kits (IBL International GmbH) according to the manufacturer's instructions. The levels of ATP, HMGB1, and cytokines (interleukin [IL]-10, IL-12, p70, tumor necrosis factor[TNF]-α, interferon[IFN]-γ, IL-6, and TNF-β) in the tumor were evaluated. Tumor tissues were collected and homogenized in a cold PBS buffer. The supernatant of the tumor homogenate was then measured using an ELISA Kit (Cytokine Kit from Biolegend) according to the manufacturer's instructions.
2.8 In vivo therapeutic efficacy of HPV gRNA–liposome + anti-PD-1 in the tumor mouse model
SiHa tumor-bearing mouse model was used as described above to study the in vivo therapeutic effects. Female hu-PBL-SCID mice aged 6 weeks were randomly divided into five groups (five in each group). On the 4th, 7th, and 10th days after tumor implantation, PBS, liposome, or HPV gRNA–liposome was injected through the tail vein in each group. The group of mice that received HPV gRNA–liposome was supplemented with an intraperitoneal injection of anti-PD-1 (100 μg in 100 μl of saline per mouse). Tumor size was measured every 2 days, and average tumor volume was calculated according to the formula: ½ (length × height × width). Six-week-old hu-PBL-SCID mice carrying SiHa tumor were randomly divided into two groups to study the effect of HPV gRNA–liposome on antitumor immune regulation in CD8+ T cell-deficient mice (n = 5 in each group). Each mouse was intraperitoneally injected with 500 μg anti-CD8α in 200 μl of saline 3 days before the administration of HPV gRNA–liposome and anti-PD-1 and then once a week. All mice were injected with HPV gRNA–liposome via caudal vein on Days 4, 7, and 10 after tumor implantation, and anti–PD-1 (100 μg in 100 μl of saline per mouse) was given intraperitoneally on Days 5, 8, and 11. The tumors were measured every 2 days, and the average tumor volume was calculated according to the formula: (length × height × width)/2.
2.9 Statistical analysis
Unless otherwise specified, all experiments were made in triplicate, and all results were expressed as mean ± standard deviation or mean ± standard error of the mean. Student t-tests or Mann–Whitney U test was used for the comparison of two groups, and a Tukey-corrected one-way analysis of variance was used for the comparison of multiple groups. All statistical analyses were performed using GraphPad Prism 7 software, and statistical significance were determined at *p < 0.05, **p < 0.01, and ***p < 0.001.
3 RESULTS
3.1 Preparation and characterization of liposome for CRISPR delivery to tumor cells
We prepared a new liposome platform. The hydrodynamic size and zeta potential of HPV–gRNA–liposomes are shown in Table 1.
| Group | Size (nm) | Zeta (mV) |
|---|---|---|
| Nanoliposome–gRNA–HPV | 146.1 ± 1.7 | 52.41 ± 2.1 |
- Abbreviations: gRNA, guide RNA; HPV, human papillomavirus.
3.2 HPV knockout by HPV gRNA–liposome induces autophagy and immunogenic cell death
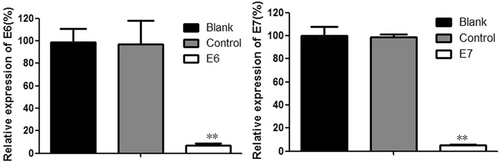
HPV gene knockout can inhibit the growth of human cervical cancer cells.9, 11 The CRISPR/Cas9 effects on HPV16 E6/E7 gene expression were determined by RT-PCR analysis after transfection of specific CRISPR/Cas9 into cells. According to our previous study,3, 9, 11 levels of HPV16 E6/E7 expression in SiHa cells transfected by the CRISPR/Cas9 targeted HPV16 were reduced, compared to the control, at 48 h. Therefore, we obtained effective gRNA which targeted HPV16 E6/E7 (Figure 1) and in our previous study,10 We established an effective CRISPR/Cas9 system to inhibit the proliferation of HPV16-positive cervical cancer SiHa cells and to induce apoptosis by inactivating the HPV16E6/E7 oncogene. Based on this system, an along-circulating pH-sensitive cationic liposome complex with excellent cell targeting and a high gene knockout rate was prepared. Liposome targeted splicing of HPV16E6/E7 was established as a basis for the treatment of HPV16-positive cervical cancer drug candidates.
Therefore, we determined whether HPV gRNA–liposome can also reduce the viability of mouse cervical cancer cells. The tumor cells were treated with different doses of PBS, naked gRNA, control liposomes, or HPV gRNA–liposomes for 48 h, and the resulting cell growth inhibition was measured (Figure 2A). The toxicity of naked HPV gRNA to all cancer cells was negligible. Control liposomes also showed limited cytotoxicity at the highest dose. In comparison, many dead cells were detected after HPV gRNA–liposome treatment.

Induced autophagy can also promote the release of DAMP and autophagosome containing tumor antigen and trigger the antigen cross-presentation process and antitumor immune response. Therefore, we hypothesized that HPV gRNA–liposomes may also induce the release of DAMP and tumor antigen, which results in ICD. The release of two ICD markers, namely, HMGB1 and ATP, to the extracellular environment was measured in SiHa cells after 48 h of treatment with HPV gRNA–liposomes to confirm our hypothesis. The release of HMGB1 or ATP in the cell culture supernatant was determined by ELISA. Compared with the control group, the HMGB1 release and ATP secretion of SiHa cells treated with HPV gRNA–liposome remarkably increased (Figure 2B,C).
3.3 Antitumor immune responses are induced by HPV gRNA–liposome in vivo
According to our previous study,3 the incidence of human cell transplantation is very high. In the experiment with LG as the main isotope, more than 80% of hu-PBL-SCID mice had detectable serum concentrations of total human LG. Similar to the previous report, the serum concentration of total human LG was variable, even amongst the SCID mice recombined with PBL of the same donor in the same experiment.
The ability of HPV gRNA–liposomes to induce ICD in tumor cells in vitro inspired us to further explore whether HPV gene knockout can activate the antitumor immune response in vivo. First, we established a subcutaneous SiHa tumor model using hu-PBL-SCID mice and measured the effect of HPV gRNA–liposome. Then, the mice were injected with HPV gRNA–liposome or naked HPV gRNA (control) through the lateral caudal vein.
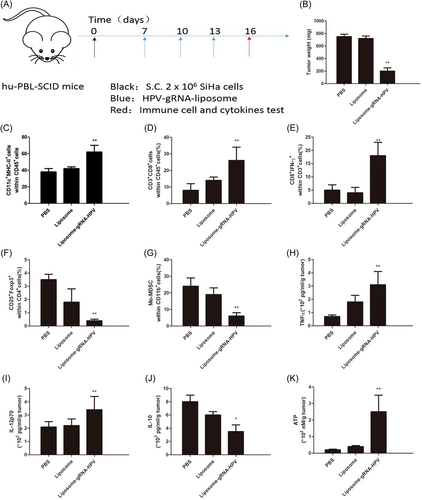
Next, we evaluated the antitumor immune response after HPV gRNA–liposome treatment. First, 2 × 106 SiHa cells were implanted subcutaneously into the left limb of mice to establish a subcutaneous tumor. When the average tumor size increased to about 50 mm3, the tumor-bearing mice were randomly divided into five groups and injected with PBS, control liposome, and HPV gRNA–liposome through the tail vein (Figure 3A). On Day 16 (2 days after the last injection), all mice were euthanized, and tumors and lymph nodes were collected to evaluate the number and phenotype of immune cells and the changes in secreted ATP, HMGB1, and cytokines. According to tumor weight, HPV gRNA–liposomes promoted tumor cell apoptosis and inhibited tumor growth (Figure 3B). We also analyzed various stimulating molecules expressed on lymph node-resident DC (LNDC) after HPV gRNA–liposome treatment. After three treatment cycles, the expression of various irritant markers, such as major histocompatibility complex II, in LNDC was upregulated (Figure 3C). Mature DCs are thought to induce antitumor immunity through the phagocytosis of tumor antigens and their presentation into T cells. The percentage of CD3+ CD8+ T cells in the tumor increased (Figure 3D). In addition, CD8+ effector T cells (CD8+ IFN-γ+) and CD8+ T-bet+ (T-box expressed in T cells) in tumors also increased compared with normal saline-treated animal tissues as assessed by flow cytometry (Figure 3E). Tregs and MDSCs play an important role in tumor immune escape, and their accumulation at tumor sites produces immunosuppressive TME. Flow cytometry showed that HPV gRNA–liposomes increased the frequency of type 1 helper T cells (CD4 + IFN-γ+) cells but decreased Treg (Foxp3 + CD25 + CD4+) and Mo-MDSC (CD11b + Ly6C + Ly6G−, Figures 3F,G), which indicates that HPV gRNA–liposome reversed the immunosuppressive TME. The comparison of cytokine release profiles (Figure 3H–J) further showed that HPV gRNA–liposomes triggered antitumor immune activation and reversed immunosuppressive TME. We also observed the expression of LC3-II in tumors isolated from HPV gRNA–liposome-treated mice. The results indicated that HPV knockout induced autophagy. In addition, the quantification of ATP release in tumor tissues by ELISA showed that PTEN expression increased after HPV gRNA–liposome treatment (Figure 3K). These results demonstrate that HPV gRNA–liposome can effectively induce autophagy and DAMP release in vivo.
3.4 HPV gRNA–liposome improve the antitumor efficacy of anti-PD-1 and confer sensitivity to anti–PD-1 in a subcutaneous mouse model
Previous studies demonstrated that HPV loss leads to poor response to anti-PD-1 therapy.3 Therefore, we explored whether the activation of antitumor immunity by HPV knockout would improve the therapeutic efficacy of ICB. First, hu-PBL-SCID mice were inoculated subcutaneously with ∼2 × 106 SiHa tumor cells on the right limb and then treated intravenously on Days 4, 7, and 10 with PBS, control gRNA, control liposome, or HPV gRNA–liposome. Some HPV gRNA–liposome-treated mice also received an intraperitoneal injection of anti-PD-1 (100 μg per mouse, 100 μl) on Days 5, 8, and 11 (Figure 4A). The combination therapy achieved greater antitumor efficacy after three cycles of treatment compared with HPV gRNA–liposome alone (p < 0.05) or anti-PD-1 alone (p < 0.01, Figure 4B). Treatment was imaged for luciferase expression throughout the study to assess tumor growth (Figure 4C).
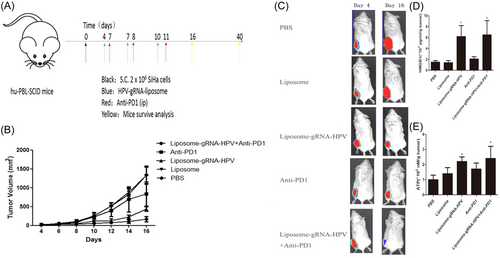
We measured the ICD biomarkers of tumors. Compared with anti-PD-1 treatment, the release of HMGB1 and ATP (Figure 4D,E) was increased by the combination treatment. All the above results suggest that HPV gRNA–liposome can effectively trigger antitumor immune responses and enhance the therapeutic efficacy of anti-PD-1 therapy.
4 DISCUSSION
Immune checkpoint inhibitors (such as anti-CTLA-4 and anti-PD-1/PD-L1) have been successful as the first-line therapy of choice for the treatment of cancer, including non-small-cell lung cancer and melanoma, which indicates that immunotherapy is a powerful treatment strategy.12, 13 However, the antitumor response rate of ICB to many cancers, including cervical cancer, is limited because of insufficient tumor immunogenicity and the existence of immunosuppressive TME.14 One strategy to improve the antitumor response of ICB is to combine it with traditional therapies, such as chemotherapy, radiotherapy, and photodynamic therapy (PDT), which may also lead to the ICD of tumor cells and initiate antitumor immunity.15, 16 However, chemotherapy and radiotherapy have serious side effects. One of the main disadvantages of PDT is the limited penetration of light into tissue. Therefore, developing additional strategies is needed to effectively and safely induce an antitumor immune response and incorporate it with ICB therapy.17, 18
The expression of some tumor suppressor genes in tumor cells, such as HPV, leads to poor response or resistance to ICB in mouse models.5, 19 Small-molecule inhibitors, such as phosphoinositide 3-kinase (PI3K) inhibitors, also have a potential role in inducing the partial recovery of tumor inhibitory function by targeting their upstream or downstream regulators. However, PI3K inhibitors have serious side effects and could not completely knock out HPV function.20 Moreover autophagy is a very important cell “housekeeper” process, which is used to degrade various misfolded proteins or cytoplasmic structures, including damaged organelles and phagocytosed pathogens. Recent evidence shows that autophagy acts as a cytoprotective mechanism against internal or external pressure and plays a crucial role in stimulating immune activation.21 Inducing the autophagy of cancer cells can also promote the secretion of DAMP and the release of tumor-associated antigens. Autophagy-deficient mice could not increase ATP release or induce antitumor immune activation. HPV is a key regulator, which negatively regulates autophagy. Cumulatively, these observations suggest that HPV knockout by HPV gRNA–liposome can induce the autophagy and DAMP release of tumor cells to trigger tumor ICD, make tumor tissues sensitive to ICB and trigger a strong and safe antitumor immune response.22-24
In this study, we developed a liposome platform for delivering HPV gRNA to tumor cells in vivo. Our results showed that the antitumor immune response of HPV gRNA–liposome was successfully triggered by inducing autophagy activation and DAMP release. In addition, HPV knockout reduced the immunosuppressive TME and improved the sensitivity of tumor cells to ICB. We compared HPV gRNA–liposome in the mouse models, anti-PD-1 drugs were used alone and their combinations and the effective antitumor effect of the combination strategy were verified.
It should be noted that this study is not clear whether restoration of other tumor suppressors might have similar effects. More efforts will also be required to further explore the mutational status of tumor suppressors and their role in immunosuppressive TME. It would be interesting to explore the effects of HPV gRNA–liposome on the proliferation and functions of immune cells, such as T cells, in future studies. Moreover, clinical results regarding the effects of loss or mutation of different tumor suppressors on therapeutic outcomes of ICB or other immunotherapies remain very limited.
Overall, our study provides a powerful strategy to stimulate an antitumor immune response. We expect that the combination of HPV gRNA–liposome drugs and ICB may lead to the development of a tumor suppressor pathway-specific immunotherapy to achieve an effective and safe cancer treatment.
AUTHOR CONTRIBUTIONS
Conceived and designed the experiments: Shuai Zhen. Performed the experiments: Shuai Zhen and Jiaojiao Lu. Analyzed the data: Shuai Zhen. Contributed reagents, materials, and analysis tools: Shuai Zhen. Wrote the paper: Shuai Zhen. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
This study was supported by grants from the National Natural Science Foundation of China (Grant No. 81602295 to Shuai Zhen). This study was supported by grants from the Key Research and Development Program of Shaanxi Province of China (2017ZDXM-SF-24-1).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The study was consistent with the principles used to relieve the pains of animals, as well as using the least number of animals possible (XBFY-2021-041).
Open Research
DATA AVAILABILITY STATEMENT
The data used to support the findings of this study are available from the corresponding author upon request.




