Prevotella as the hub of the cervicovaginal microbiota affects the occurrence of persistent human papillomavirus infection and cervical lesions in women of childbearing age via host NF-κB/C-myc
Binhua Dong, Yuxuan Huang, and Hongning Cai contributed equally to this study.
Abstract
There is evidence that coinfection of cervicovaginal high-risk human papillomavirus (HR-HPV) and bacteria is common in women of childbearing age. However, the relationship between bacterial vaginosis (BV) and persistent HR-HPV infection in women of childbearing age and the underlying mechanisms remain unclear. In this study, we determined whether BV affects persistent HR-HPV infection in women aged 20–45 years and explored the possible mechanisms of their interactions. From January 1 to April 30, 2020, we recruited women aged 20–45 years with and without BV at a ratio of 1:2 from Fujian Maternity and Child Health Hospital. All women were followed up at 0, 12, and 24 months. A BV assay, HR-HPV genotyping and cervical cytology were performed at each follow-up. At 0 months, additional vaginal secretions and cervical exfoliated cells were collected for 16S ribosomal RNA sequencing, bacterial metabolite determination, and POU5F1B, C-myc, TLR4, NF-κB, and hTERT quantification. A total of 920 women were included. The abundance of Prevotella (p = 0.016) and Gardnerella (p = 0.027) were higher, whereas the abundance of Lactobacillus was lower (p = 0.001) in women with persistent HR-HPV infection and high-grade squamous intraepithelial lesions (HSIL). The abundance of Prevotella (p = 0.025) and Gardnerella (p = 0.018) increased in the vaginas of women with persistent HPV16 infection, whereas only the abundance of Prevotella (p = 0.026) was increased in women with persistent HPV18 infection. The abundance of Prevotella in the vagina was significantly positively correlated with the expression levels of TLR4, NF-κB, C-myc, and hTERT in host cervical cells (p < 0.05). Our findings suggest that overgrowth of Prevotella in the vagina may influence the occurrence of persistent HR-HPV infection-related cervical lesions through host NF-κB and C-myc signaling.
1 INTRODUCTION
The incidence of high-risk human papillomavirus (HR-HPV) infection is high in women of childbearing age, and recent studies have found that the incidence of persistent HR-HPV infection-related cervical cancer or precancerous lesions is high at younger ages.1, 2 There is also evidence that coinfection with HR-HPV and other vaginal bacteria is common, especially in sexually active women of childbearing age.3, 4 Women with bacterial vaginosis (BV) are more prone to vaginal coinfection,5 and cervical HR-HPV infection is more common in women with BV,4 but the relationship between BV and persistent HR-HPV infection in women of childbearing age remains unclear.
The microbiota composition plays a critical role in the development of chronic diseases, especially in female genital tract infectious diseases.3 The cervicovaginal microenvironment comprises the microbiota and its metabolites, and the cervicovaginal microbiota can be classified into five community state types (CSTs). CST-I, -II, -III, and -V are dominated by Lactobacillus spp. and mainly protect the mucosa, whereas CST-IV is dominated by various anaerobic bacteria, including Gardnerella, Prevotella, and Atopobium spp.6-9 Changes in the cervicovaginal microbiota may be associated with HR-HPV infection-associated cervical cancer.10 Some genital tract BV-related bacteria may also be associated with neoplasms, including Atopobium vaginae, Prevotella, Gardnerella, Sneathia sanguinegens and Fusobacterium.8, 10 Patients with HR-HPV-associated cervical lesions showed a decrease in normally dominant Lactobacillus populations and an increase in Gardnerella, unlike healthy women.11, 12 Furthermore, Gardnerella vaginalis and Prevotella were found to be associated with cervical intraepithelial neoplasia (CIN) persistence and slower regression.13 However, the interactions between BV and persistent HR-HPV infection in women of childbearing age remain unclear.
Cancer is a metabolic disease, and bacterial functional metabolites in the cervicovaginal microenvironment have the potential to reveal the interrelationship between host and BV-related bacteria during HR-HPV infection and cancer progression.12 Previous studies have suggested that an imbalance in the vaginal microenvironment plays an important role in the development of HR-HPV infection.12 Cervicovaginal pH, cervicovaginal hygiene, and the concentrations of β-glucuronidase, neuraminidase, sialidase, and leukocyte esterase (LE) serve as important markers of vaginal microenvironment function.14 It has also been reported that G. vaginalis can secrete sialidase, higher concentrations of which are associated with an increased risk of cervical lesions.13, 15 However, the relationship between vaginal microenvironment function and persistent HR-HPV infection remains unclear.
The mechanism through which BV influences HR-HPV-associated cervical lesions has attracted attention in recent years. A previous study has suggested that tumor-related genes play an important role in HR-HPV infection progressing into cervical cancer.16 Subsequent studies have revealed that TLR2/4/9 expression shows positive correlations with progression.17-19 Altoos et al.20 demonstrated that the expression level of NF-κBp65 is associated with poor clinical outcomes in patients with locally advanced cervical cancer. Another study revealed that the expression of C-myc and POU5F1B is higher in CIN grade 2 or worse (CIN2+) than in normal patients or CIN1.21, 22 hTERT expression also increases in CIN2+.23
Although these findings suggest that BV may play an important role in the progression of HR-HPV infection to cervical cancer, the relationship between cervicovaginal microbiota and persistent HR-HPV infection in women of childbearing age and the underlying mechanism has not yet been clearly studied. The aim of this study was to identify whether BV affects persistent HR-HPV infection in women aged 20–45 years and explore the possible mechanisms of their interaction.
2 MATERIALS AND METHODS
2.1 Study design and sample collection
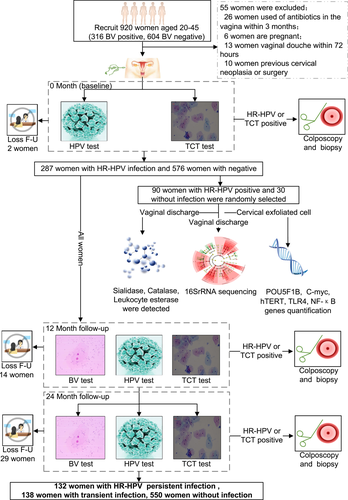
All participants were recruited at Fujian Maternity and Child Health Hospital between January 1 and April 30, 2020. Eligible participants satisfied the following criteria: (1) 20–45 years of age; (2) underwent vaginal microecological testing for BV screening; and (3) underwent primary cervical cancer screening in the past 3 years. The exclusion criteria were: (1) pregnancy; (2) vaginal douche within 72 h; (3) sexual intercourse within 3 days before screening; (4) use of antibiotics in the vagina in the past 3 months; (5) history of hysterectomy; and (6) previous cervical neoplasia. Vaginal secretions and cervical exfoliated cell samples were collected from all eligible participants for BV testing, HR-HPV genotyping and cervical cytology at the time of recruitment, 12 months and 24 months later. At 0 months, vaginal fornix secretions were collected from 120 randomly selected eligible participants (90 HR-HPV-positive and 30 HR-HPV-negative) for 16S ribosomal RNA (rRNA) sequence analysis, vaginal microbial metabolite detection, and host gene expression assays. The vaginal secretion samples were collected from the posterior vaginal fornix with a swab (Figure 1). The swab was placed into a collection tube (Kangjian Medical Supplies Co.) and immediately stored at −80°C. Cervical exfoliated cells were collected from the cervical canal using plastic brushes, placed in ThinPrep® Pap Test PreservCyt® Solution (Hologic Inc.), and immediately stored at 2–8°C for cytology and HR-HPV genotyping within 7 days. The study was approved by the Ethics Committee of Fujian Maternity and Child Health Hospital (2020KY015), and written informed consent was obtained from all participants. The study was registered under ChiCTR2000032981 at http://www.chictr.org.cn.
2.2 16S rRNA gene sequencing and analysis
Genomic DNA was extracted using the E.Z.N. A™ Mag-Bind Soil DNA Kit (Omega Bio-Tek) according to the manufacturer's instructions. The samples were quantified and pooled in equimolar ratios using the Qubit 3.0 DNA Kit (Invitrogen). The primers (Nobar_341F: 5ʹ-CCTACGGGNGGCWGCAG-3ʹ; and Nobar_805R: 5ʹ-GACTACHVGGGTATCTAATCC-3ʹ) directionally targeting the V3 and V4 hypervariable regions, respectively, of the 16S rRNA gene were used. To differentiate individual samples and obtain accurate phylogenetic and classification information, forward and reverse error correction barcodes were added to gene products. Purified polymerase chain reaction (PCR) products were quantified using the Qubit 3.0 Fluorometric High-Sensitivity dsDNA Assay Kit (Invitrogen) before library construction using the KAPA LTP Library Preparation Kit (Kapa Biosystems). Samples of normalized equimolar concentrations of each amplicon were pooled on the MiSeq PE300 sequencing platform (Illumina) at Sangon Biotech. High-throughput amplicon sequencing of 2 × 300 paired-end reads was conducted on an Illumina MiSeq platform (Illumina). Raw FASTQ files were obtained and merged. Operational taxonomic units (OTUs) were clustered with a cut-off value of 97% using the CD-HIT online tool (v. 4.6.1). Representative sequences of each OTU were classified, and taxonomic data were assigned to each representative sequence using the Ribosomal Database Project (RDP, v. 11.5). QIIME software was used to evaluate the α-diversity and β-diversity of each sample. Principal coordinate analysis (PCoA) was also used to evaluate microbial β-diversity among samples.
2.3 Vaginal microbiological metabolite detection
Vaginal secretion samples were used to detect hydrogen peroxide, sialidase, LE, β-glucuronidase, and β-N-acetylamino-glucosidase levels with a bPR-2014A vaginitis automatic detector and supporting detection kit (Master Biotechnology Co., Ltd.). Hydrogen peroxide was detected by peroxidase hydrolysis. The sialidase assay was performed using enzymatic hydrolysis of 5-bromo-4-chloro-3-indole neuraminidase. LE was detected using the hydrolyzed acetate method. The β-glucuronidase assay used hydrolysis of 5-bromo-4-chloro-3-indolyl-β-d glucuronide cyclohexylamine salt. β-N-acetylamino-glucosidase was detected by enzymatic hydrolysis. All laboratory procedures were performed according to the manufacturer's instructions.
2.4 HR-HPV genotyping
HR-HPV genotyping was performed to detect HR-HPV genotypes according to the manufacturer's instructions (Yaneng Bioscience Co., Ltd.), as described previously.24
2.5 Cervical cytology
All liquid-based cytology specimens were analyzed using the Bethesda System (TBS), independently by two experienced cytopathologists. The results were classified based on the TBS grading system as cervical atypia squamous cells with unclear significance, atypical squamous cells-cannot rule out high-grade lesion, low-grade squamous intraepithelial lesion (LSIL), high-grade squamous intraepithelial lesion (HSIL), squamous cervical cancer, atypical glandular cells and adenocarcinoma.
2.6 PCR of signaling pathway genes
Total RNA was extracted from cervical epithelial cells using TRIzol® reagent (Life Technologies), and total RNA from cervical epithelial cells was reverse transcribed to complementary DNA using GoScriptTM Reverse Transcription Kit (Promega). DNA was PCR-amplified using primers for C-myc, hTERT, TLR4, NF-κB, and POU5F1B. The PCR primers were shown in Supporting Information: Table S1. The messenger RNA expression levels in each sample were normalized to the respective GAPDH expression levels and calculated using the method.
2.7 Histology
Women who were HR-HPV positive and/or had abnormal cytological results were referred for colposcopy and punch biopsy. Women with a punch biopsy diagnosis of ≥HSIL were subjected to a loop electrosurgical excision cone biopsy or conization procedure carried out using a cold knife. The specimens were fixed in 10% formalin and processed for paraffin embedding. Subsequently, 4-μm-thick histological sections were cut and stained with hematoxylin–eosin. The cervical biopsy specimens were histologically examined and classified according to the CIN system.6
2.8 Statistical analysis
To evaluate the relationships between cervicovaginal microbiota, HR-HPV infection, and cervical lesions, samples were divided into four subgroups: HR-HPV negative (−) and pathology = normal/CIN1 (−); HR-HPV-transient infection (TF) and pathology (−); HR-HPV-persistent infection (PF) and pathology (−); and HR-HPV-PF and pathology = CIN2+ (+). To further investigate the relationship among cervicovaginal microbiota, different HR-HPV genotypes, and cervical lesions, samples were further divided into 11 subgroups as follows: HR-HPV (−); HPV-16-TF; HPV-16-PF; HPV-18-TF; HPV-18-PF; HPV-A9-TF; HPV-A9-PF; HPV-A7-TF; HPV-A7-PF; HPV-A5/6-TF; and HPV-A5/6-PF. All samples also divided into six subgroups as follows: HR-HPV (−) and cytology normal (−) and pathology (−); HR-HPV (−) and cytology abnormal (+) and pathology (−); HR-HPV (+) and cytology (−) and pathology (−); HR-HPV (+) and cytology (−) and pathology (+); HR-HPV (+) and cytology (+) and pathology (−); and HR-HPV (+) and cytology (+) and pathology (+). Data are expressed as the mean ± standard deviation. χ2 tests and Fisher's exact tests were used to assess the associations between cervicovaginal microbiota variables and HR-HPV infection. SPSS v24.0 (IBM) and R statistical software v3.6.3 (Mathsoft) were used for all statistical analyses. Mothur software v1.30.1 and the SILVA rRNA database were used for OTU, α-diversity and β-diversity analyses of microbiota. All tests were two-sided, and results with p < 0.05 were considered statistically significant.
3 RESULTS
3.1 Prospective follow-up analysis of 920 participants identifies associations between BV and persistent HR-HPV genotype infection
Previous studies have shown that the incidence of BV25, 26 in women aged 20–45 years ranged from 29.2% to 38.5%, and the HR-HPV infection rate27 in China was as high as 18.9%. Women with BV may also have a higher risk of HR-HPV infection.10, 12, 25 However, the relationship between persistent infection of different genotypes of HR-HPV and BV remains unclear. To address this major issue, a 2-year prospective cohort study was performed including 920 women aged 20–45 years with different BV statuses (BV positive and BV negative). The characteristics of the recruited participants are displayed in Supporting Information: Table S2. Fifty-five women were excluded based on the exclusion criteria, and 865 eligible participants were included in the analysis. The incidence of BV in the recruited subjects was 34.3% (316/920), and the infection rate of HR-HPV was 31.2% (287/920) at the time of enrollment. The 2-year persistent HR-HPV infection rate was 16.1% (132/820). There was no difference in HR-HPV, HPV16, and HPV18 infection rates between patients with and without BV, and differences only existed in other specific genotypes, such as HPV33 (p = 0.034), HPV51 (p = 0.034), and HPV59 (p = 0.034). The persistent infection rate of HR-HPV was significantly higher in women with BV than in women without (75.5% vs. 31.7%, p < 0.001). Regarding specific genotypes, women with BV have higher rates of persistent infection with HPV16, HPV18, HPV31, HPV45, HPV51, HPV52, HPV56, HPV58, HPV59, and HPV66 (p = 0.038, p = 0.019, p = 0.076, p = 0.133, p = 0.020, p = 0.001, p = 0.001, p = 0.001, p = 0.020, and p = 0.022, respectively) (Figure 1 and Table 1).
| Variables | BVa positive (n = 306) | BV negative (n = 514) | p Value |
|---|---|---|---|
| HR-HPV negative | 65.4% (200/306) | 68.1% (350/514) | 0.420 |
| HR-HPV positive | 34.6% (106/306) | 31.9% (164/514) | |
| HPV16 negative | 92.8% (284/306) | 92.6% (476/514) | 0.914 |
| HPV16 positive | 7.2% (22/306) | 7.4% (38/514) | |
| HPV18 negative | 96.7% (296/306) | 93.8% (482/514) | 0.063 |
| HPV18 positive | 3.3% (10/306) | 6.2% (32/514) | |
| HPV31 negative | 98.7% (302/306) | 98.8% (508/514) | 1.000 |
| HPV31 positive | 1.3% (4/306) | 1.2% (6/514) | |
| HPV33 negative | 96.1% (294/306) | 98.4% (506/514) | 0.034 |
| HPV33 positive | 3.9% (12/306) | 1.6% (8/514) | |
| HPV35 negative | 98.0% (300/306) | 99.6% (512/514) | 0.065 |
| HPV35 positive | 2.0% (6/306) | 0.4% (2/514) | |
| HPV39 negative | 98.7% (302/306) | 98.4% (506/514) | 1.000 |
| HPV39 positive | 1.3% (4/306) | 1.6% (8/514) | |
| HPV45 negative | 99.3% (304/306) | 98.4% (506/514) | 0.418 |
| HPV45 positive | 0.7% (2/306) | 1.6% (8/514) | |
| HPV51 negative | 98.7% (302/306) | 96.1% (494/514) | 0.034 |
| HPV51 positive | 1.3% (4/306) | 3.9% (20/514) | |
| HPV52 negative | 89.5% (274/306) | 93.0% (478/514) | 0.083 |
| HPV52 positive | 10.5% (32/306) | 7.0% (36/514) | |
| HPV56 negative | 97.4% (298/306) | 97.7% (502/514) | 0.802 |
| HPV56 positive | 2.6% (8/306) | 2.3% (12/514) | |
| HPV58 negative | 95.4% (292/306) | 96.5% (496/514) | 0.443 |
| HPV58 positive | 4.6% (14/306) | 3.5% (18/514) | |
| HPV59 negative | 98.7% (302/306) | 96.1% (494/514) | 0.034 |
| HPV59 positive | 1.3% (4/306) | 3.9% (20/514) | |
| HPV66 negative | 99.3% (304/306) | 98.4% (506/514) | 0.418 |
| HPV66 positive | 0.7% (2/306) | 1.6% (8/514) | |
| HPV68 negative | 98.0% (300/306) | 98.8% (508/514) | 0.539 |
| HPV68 positive | 2.0% (6/306) | 1.2% (6/514) | |
| HR-HPV transient infection | 24.5%(26/106) | 68.3% (112/164) | <0.001 |
| HR-HPV persistent infection | 75.5%(80/106) | 31.7% (52/164) | |
| HPV16 transient infection | 22.7% (5/22) | 50.0% (19/38) | 0.038 |
| HPV16 persistent infection | 77.3% (17/22) | 50.0% (19/38) | |
| HPV18 transient infection | 20.0% (2/10) | 68.8% (22/32) | 0.019 |
| HPV18 persistent infection | 80.0% (8/10) | 31.2% (10/32) | |
| HPV31 transient infection | 0.0% (0/4) | 66.7% (4/6) | 0.076 |
| HPV31 persistent infection | 100.0% (4/4) | 33.3% (2/6) | |
| HPV33 transient infection | 33.3% (4/12) | 50.0% (4/8) | 0.648 |
| HPV33 persistent infection | 66.7% (8/12) | 50.0% (4/8) | |
| HPV35 transient infection | 66.7% (4/6) | 100.0% (2/2) | 1.000 |
| HPV35 persistent infection | 33.3% (2/6) | 0.0% (0/2) | |
| HPV39 transient infection | 100.0% (4/4) | 75.0% (6/8) | 0.515 |
| HPV39 persistent infection | 0.0% (0/4) | 25.0% (2/8) | |
| HPV45 transient infection | 0.0% (0/2) | 75.0% (6/8) | 0.133 |
| HPV45 persistent infection | 100.0% (2/2) | 25.0% (2/8) | |
| HPV51 transient infection | 0.0% (0/4) | 70.0% (14/20) | 0.020 |
| HPV51 persistent infection | 100.0% (4/4) | 30.0% (6/20) | |
| HPV52 transient infection | 31.3% (10/32) | 72.2% (26/36) | 0.001 |
| HPV52 persistent infection | 68.8% (22/32) | 27.8% (10/36) | |
| HPV56 transient infection | 0.0% (0/8) | 83.3% (10/12) | 0.001 |
| HPV56 persistent infection | 100.0% (8/8) | 16.7% (2/12) | |
| HPV58 transient infection | 0.0% (0/14) | 55.6% (10/18) | 0.001 |
| HPV58 persistent infection | 100.0% (14/14) | 44.4% (8/18) | |
| HPV59 transient infection | 0.0% (0/4) | 70.0% (14/20) | 0.020 |
| HPV59 persistent infection | 100.0% (4/4) | 30.0% (6/20) | |
| HPV66 transient infection | 0.0% (0/2) | 100.0% (8/8) | 0.022 |
| HPV66 persistent infection | 100.0% (2/2) | 0.0% (0/8) | |
| HPV68 transient infection | 33.3% (2/6) | 33.3% (2/6) | 1.000 |
| HPV68 persistent infection | 66.7% (4/6) | 66.7% (4/6) | |
| Cervical histopathology | |||
| NILM/LSIL (CIN1) | 92.2% (282/306) | 91.1% (468/514) | 0.583 |
| HSIL or worse | 7.8% (24/306) | 8.9% (46/514) |
- Abbreviations: BV, bacterial vaginosis; CIN, cervical intraepithelial neoplasia; HR-HPV, high-risk human papillomavirus; HSIL, high-grade squamous intraepithelial lesion; LSIL, low-grade squamous intraepithelial lesion; NILM, negative for intraepithelial lesion or malignancy.
- a Used Nugent scoring system to diagnose BV.
3.2 Prevotella was closely related to persistent HR-HPV infections
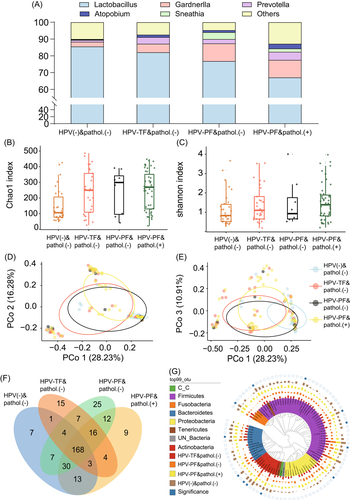
Knowing that women with BV have a higher probability of persistent HR-HPV infection, we hypothesized that different BV-associated bacteria may play a role in persistent infection with different HR-HPV genotypes. To test this hypothesis, a total of 120 women were randomly selected from the 920 participants and cervicovaginal secretions were collected for 16S rRNA sequencing at the time of recruitment. During the 2-year follow-up, 5 women were excluded because of the lack of follow-up data or because the samples were substandard; thus, 115 samples were subjected to 16S rRNA analysis (Figure 1). The accumulation curve for microbial species showed that the number of samples was sufficient (Supporting Information: Figure S1A), and the sequencing depth of most of the samples was over 50 000 (Supporting Information: Figure S1B). The highest abundance at the phylum level in the cervicovaginal region was observed for Firmicutes (71.49%), Actinomycetes (15.44%), and Bacteroidetes (5.89%). At the genus level, the most abundant bacteria were Lactobacillus (65.82%), Gardnerella (11.5%), Prevotella (5.02%), Sneathia (2.57%), and Atopobium (1.87%), which belong to the Firmicutes, Actinomycetes, Bacteroidetes, Fusobacteria, and Actinomycetes phyla, respectively (Supporting Information: Figure S1C,D). There was a negative correlation between the abundance of Actinobacteria and Firmicutes at the phylum level (r = −0.58, p < 0.01). Similarly, the abundance of Prevotella (r = −0.51, p < 0.01) and Gardnerella (r = −0.32, p = 0.01) was negatively correlated with presence of Lactobacillus (Supplementary Figure S1E,F). The abundance of Prevotella (6.10% vs. 0.02%, p = 0.003) and Gardnerella (11.61% vs. 2.01%, p = 0.024) in women with HR-HPV-persistent infection (PF) and pathology (+) (CIN2 or worse) was higher than that in women with HR-HPV negative (−) and pathology (−) (cervical epithelium has no lesion or only low-grade lesion). However, the abundance of Lactobacillus in women with HR-HPV-PF and pathology (+) was lower than that in women with HR-HPV (−) and pathology (−) (61.03% vs. 88.8%, p = 0.001; Figure 2A,G). The α-diversity analysis showed that women with HR-HPV-transient-infection (TF) and pathology (+) had a higher Chao index than women with HR-HPV (−) and pathology (−) (p = 0.008). However, there was no significant difference in the Shannon index among the groups (p = 0.090) (Figure 2B,C). The PCoA of the samples revealed that the microbiota of women with HR-HPV (−) and pathology (−) was significantly different from that of women with HR-HPV-TF and pathology (−) as well as HR-HPV-TF and pathology (+) (Figure 2D,E). The abundances of Prevotella (p = 0.016, p = 0.015, p = 0.029) and Gardnerella (p = 0.038, p = 0.011, p = 0.029) in woman with HR-HPV (+) and TCT (−) and pathology (+), HR-HPV (+) and TCT (+) and pathology (−), and HR-HPV (+) and TCT (+) and pathology (+) were higher than that in women with HR-HPV (+) and TCT (−) and pathology (−) (Supporting Information: Figure S2). Based on the above findings, women with vaginocervical microbiota dominated by Gardnerella and/or Prevotella, or with decreased Lactobacillus abundance, are more likely to develop HR-HPV persistent infection.
3.3 Cervicovaginal Prevotella dominance increases the likelihood of persistent infection with HPV16 and HPV18
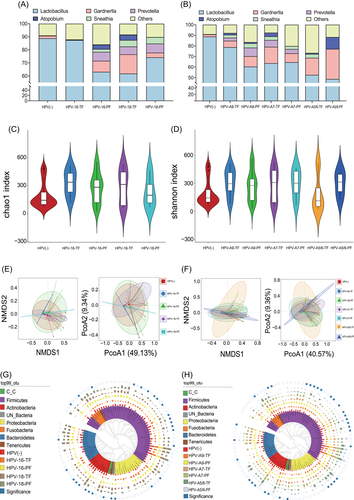
The impact of cervicovaginal bacterial abundance changes on persistent HR-HPV infection has been demonstrated, so is there any difference in the effect on persistent infection of separate HR-HPV genotypes? The previous evidence was inadequate, so we further evaluated the impact of cervicovaginal microbiota on persistent infection of different HR-HPV genotypes. The results revealed that women with HPV-16-PF had significantly higher abundances of Prevotella (6.83% vs. 0.13%, p = 0.025) and Gardnerella (8.42% vs. 0.22%, p = 0.018) than women with HPV-16-TF, whereas the abundance of Lactobacillus was significantly reduced (87.45% vs. 51.71%, p = 0.026). On the contrary, women with HPV-18-PF showed a higher abundance of Prevotella (6.94% vs. 4.96%, p = 0.026) than women with HPV-18-TF. However, the abundance of Gardnerella in women with HPV-18-PF was lower than that in women with HPV-18-TF (3.59% vs. 14.74%, p = 0.039) (Figure 3A,G). The abundance of Prevotella was higher (11.63% vs. 2.91%, p = 0.035) in women positive for HPV- species9 (A9)-PF than that in women with HPV-A9-TF, and the abundance of Lactobacillus (48.64% vs. 78.77%, p = 0.020) was significantly reduced. Although the abundance of Gardnerella (9.85% vs. 6.03%, p = 0.073) was higher in women with HPV-A9-PF than in women with HPV-A9-TF, there was no significant difference. It is noteworthy that the abundances of Prevotella, Gardnerella, and Lactobacillus in women with HPV-species7 (A7)-PF were not significantly different than that of women with HPV-A7-PF (Prevotella: p = 0.434, Gardnerella: p = 0.582, Lactobacillus: p = 0.692). In women with HPV-species5/6 (A5/6)-PF, the abundance of Gardnerella (p = 0.037) and Atopobium (p = 0.003) was higher than that in women with HPV-A5/6-TF (Figure 3B,H). Based on the above evidence, women with increased cervicovaginal Prevotella abundance are more likely to develop a persistent infection with HPV16 and HPV18.
3.4 Predictive analysis of the function of differential cervicovaginal microbiota
To understand which pathway functions are involved in differential cervicovaginal microbiota, especially Prevotella, the study further performed functional predictions. It was found that in women with HR-HPV-PF and pathology (+), overgrowth of bacteria in the cervicovaginal region activated nucleotide-binding and oligomerization domain (NOD)-like receptor signaling, p53 signaling, and steroid hormone biosynthesis pathways to a greater extent (all p < 0.001). Similarly, women with HPV16-PF showed increased activation of p53 signaling and NOD-like receptor signaling pathways (all p < 0.001). There were significant differences in the calcium signaling (p < 0.001), NOD-like receptor signaling (p = 0.012), and the p53 signaling pathways (p = 0.004) between women with HR-HPV (–) and TCT (−) and pathology (−) and those with HR-HPV (+) and TCT (−) and pathology (+). The results indicate that the NOD-like receptor signaling and p53 signaling pathways may be involved in the promotion of persistent HR-HPV infection by Prevotella overgrowth (Figure 4).
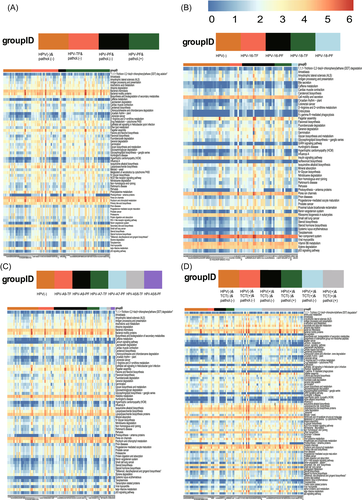
3.5 Correlation of differential cervicovaginal microbiota, microbiological metabolites, and host gene expression in women with persistent HR-HPV infection
To clarify the relationship between the above differential microbiota, metabolites, NOD-like receptor signaling pathway, and p53 signaling pathway, metabolite and genetic testing were performed in 120 women who underwent 16S RNA sequencing. The metabolites included hydrogen peroxide, sialidase, LE, β-glucuronidase, and β-N-acetylamino-glucosidase, and genes included TLR4, NF-κB, C-myc, hTERT, and POU5F1B. By comparing the effect of cervicovaginal microbiota on the expression of host genes, Prevotella abundance was found to be positively correlated with TLR4, NF-κB, C-myc, hTERT, and POU5F1B expression (r2 = 0.98, 0.96, 0.92, 0.90, and 0.84, respectively; all p < 0.001). Meanwhile, Lactobacillus (r2 = −0.46, −0.39, −0.41, −0.38, and −0.38, respectively; all p < 0.001) and Gardnerella (r2 = −0.43, −0.37, −0.26, −0.45, and-0.29, respectively; p < 0.001, p < 0.001, p = 0.109, p < 0.001, p = 0.057, respectively) showed a negative correlation with the aforementioned genes (Figure 5A,B). When the relationship between cervicovaginal microbiota and vaginal metabolites was analyzed, we found that Prevotella (r2 = 0.55, p = 0.041) abundance and Gardnerella (r2 = 0.68, p = 0.033) abundance were positively correlated with sialidase concentration, but not with the other metabolites (Figure 5C). This evidence suggests that women with Prevotella predominance may show increased activation of cervical TLR4, NF-κB, C-myc, and hTERT expression by secreting sialidase, which can contribute to progression to persistent HR-HPV infection.
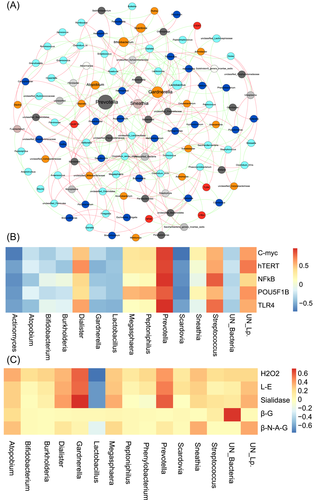
3.6 Validation of cervicovaginal microbial metabolites and host gene expression in women with different HR-HPV-PF genotypes
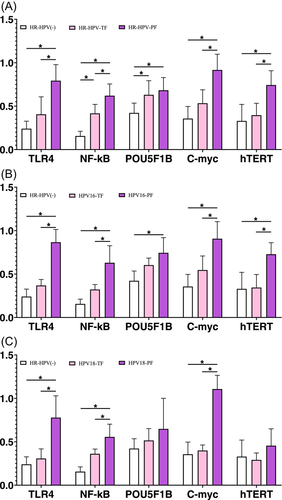
To further test the above hypothesis, at 0 months, all women with complete follow-up were tested for TLR4, NF-κB, C-myc, hTERT, POU5F1B, H2O2, sialidase, and LE concentrations, 820 qualified participants were included for validation. We found that women who developed persistent HR-HPV infection within 24 months had higher TLR4, NF-κB, C-myc, and hTERT expression at recruitment (Figure 6A, all p < 0.001). Women with persistent HPV16 infection within 24 months also showed higher expression of the above four genes (p = 0.002, p = 0.013, p = 0.048, p = 0.024, respectively; Figure 6B). However, women with persistent HPV18 infection showed increased expression of only TLR4, NF-κB, and C-myc (p = 0.002, p = 0.015 and p < 0.001, respectively; Figure 6C). In women with persistent HR-HPV, HPV16, and HPV18 infections, only the concentration of sialidase increased among the measured metabolites (p < 0.001, p = 0.009, p = 0.019, respectively; Table 2). These findings indicate that the predominance of Prevotella in the cervicovaginal region promotes persistent infection of HR-HPV, HPV16 and HPV18, and that this effect is related to high concentration/expression of sialidase, TLR4, NF-κB, C-myc, and hTERT.
| Variables | Hydrogen peroxide | p Value | Sialidase | p Value | Leukocyte esterase | p Value | |||
|---|---|---|---|---|---|---|---|---|---|
| Negative (n = 84) | Positive (n = 186) | Negative (n = 190) | Positive (n = 80) | Negative (n = 69) | Positive (n = 201) | ||||
| HR-HPV TF % (n/N, n = 138) | 54.8 (46/84) | 49.5 (92/186) | 0.420 | 63.2 (120/190) | 22.5 (18/80) | <0.001 | 69.6 (48/69) | 44.8 (90/201) | <0.001 |
| HR-HPV PF % (n/N, n = 132) | 45.2 (38/84) | 50.5 (94/186) | 36.8 (70/190) | 77.5 (62/80) | 30.4 (21/69) | 55.2 (111/201) | |||
| HPV16 TF % (n/N, n = 24) | 45.5 (10/22) | 36.8 (14/38) | 0.512 | 50.0 (22/44) | 12.5 (2/16) | 0.009 | 36.4 (4/11) | 40.8 (20/49) | 1.000 |
| HPV16 PF % (n/N, n = 36) | 54.5 (12/22) | 63.2 (24/38) | 50.0 (22/44) | 87.5 (14/16) | 63.6 (7/11) | 59.2 (29/49) | |||
| HPV18 TF % (n/N, n = 24) | 57.1 (8/14) | 57.1 (16/28) | 1.000 | 68.8 (22/32) | 20.0 (2/10) | 0.019 | 53.3 (8/15) | 59.3 (16/27) | 0.710 |
| HPV18 PF% (n/N, n = 18) | 42.9 (6/14) | 42.9 (12/28) | 31.2 (10/32) | 80.0 (8/10) | 46.7 (7/15) | 40.7 (11/27) | |||
- Abbreviations: HR-HPV, high-risk human papillomavirus; PF, persistent infection; TF, transient infection.
4 DISCUSSION
Previous studies have suggested that the cervicovaginal microbiota plays an essential role in the health of women.28 Cervicovaginal microbiota influences HR-HPV infection and even promotes cervical lesions.3, 13 However, other evidence25 suggests that HR-HPV contributes to an imbalance in the cervicovaginal microbiota through its negative effects on Lactobacilli. Previous reports have linked BV to HR-HPV infection.29 However, it is still not clear whether BV affects the persistent infection of HR-HPV, especially the relationship between BV and persistent infection of different HR-HPV genotypes. There is no report on the mechanisms of BV involvement in persistent HR-HPV infection.
In the present, we found that the abundances of Lactobacillus, Gardnerella, Prevotella, and Atopobium were strongly associated with persistent HR-HPV infection. The decrease in Lactobacillus and the increases in Prevotella, Gardnerella, and Atopobium abundance were closely related to persistent HR-HPV infection and cervical lesions. A recent study also showed that in women with non-Lactobacillus dominance, a twofold increase in the risk of HR-HPV infection was observed than in those with Lactobacillus crispatus dominance.28 Depletion and the presence of BV-related bacteria, including Prevotella and Gardnerella, are associated with CIN2 persistence and slower regression.13 Previous studies30, 31 have shown that the overgrowth of Prevotella can produce polyamines, increase vaginal pH, inhibit Lactobacillus growth, and promote the overgrowth of G. vaginalis. In addition, the overgrowth of G. vaginalis produces excess amino acids. These amino acids can be utilized by Prevotella and eventually cause coreproduction, resulting in changes in the microenvironment of the lower genital tract, which can cause lesions. Women with Gardnerella, Prevotella, and/or Atopobium co-overgrowth in our study had a higher rate of HR-HPV persistent infection. Based on previous evidence and our study, it was found that Lactobacillus, Gardnerella, and Prevotella play important roles in the occurrence of persistent HR-HPV infection.
Persistent infection with HPV16 and HPV18 are the main causes of cervical cancer. HPV16 is closely related to cervical squamous cell carcinoma, and HPV18 is closely related to cervical adenocarcinoma.32 It is still unclear whether BV-related bacteria have the same relationship to persistent infection with HPV16 and HPV18. Our study found that abundance changes of Lactobacillus, Prevotella, Gardnerella, and Atopobium were closely related to persistent infection with HPV16, but only Lactobacillus and Prevotella were related to persistent infection with HPV18. Studies33 have found that Prevotella can secrete collagenase and fibrinolysins, which can damage cervicovaginal epithelial cells, destroy the stability of the microenvironment, and promote the persistence of other microbial infections, such as viruses. This also verifies that the overgrowth of Prevotella is associated with persistent infection with both HPV16 and HPV18, while other BV-associated bacteria are not.
Studies34, 35 have shown that the mechanisms of bacterial carcinogenesis are often via overgrowth, activation of TLR4/NF-κB, inhibition of apoptosis of infected cells, and cell carcinogenesis. Other studies36, 37 have also shown that NF-κB overexpression due to microbial infection further activates hTERT, resulting in the immortalization of infected cells. Moreover, microorganisms activate C-myc via NF-κB, which again further activates hTERT, resulting in the malignant transformation of cells.38, 39 Previous studies have reported that excessive proliferation of Prevotella can activate NF-κB. This study further explored the relationship between the overgrowth of Prevotella in the vagina and TLR4, NF-κB, C-myc, and hTERT in cervical epithelial cells. The results found that the abundance of Prevotella had positive correlations with TLR4, NF-κB, C-myc, and hTERT expression, whereas Lactobacillus abundance showed negative correlations with these genes. Moreover, the abundance of Prevotella is related to the high concentration of sialidase. We further verified the relationship between the above genes and HR-HPV persistent infection in a larger population and found that women with high expression of TLR4, NF-κB, and C-myc had higher HR-HPV, HPV16 and HPV18 persistent infection rates within 2 years. Prevotella may affect TLR4, NF-κB and C-myc expression, resulting in persistent HR-HPV infection and progression to related cervical lesions.
There are some limitations to our study. First, this study only followed-up subjects for 2 years, and data on persistent HR-HPV infection for longer periods of time are unavailable. Subjects will continue to be followed up to further elucidate the relationship between Prevotella overgrowth and long-term HR-HPV infection. Second, the mechanisms by which Prevotella promotes TLR4/NF-κB activation are unclear, and the mechanism by which NF-κB activates hTERT, which ultimately leads to persistent HR-HPV infection, is also unknown. At present, we have established an in vitro coculture system of Prevotella and HR-HPV-infected cells, and the specific interactions between them will be analyzed in detail.
5 CONCLUSIONS
Our findings suggest that cervicovaginal microbiota composition may be a predictive biomarker of persistent HR-HPV infection in women of childbearing age. The overgrowth of Prevotella is closely related to persistent infection with HPV16 and HPV18. Prevotella in the vagina may influence the occurrence of persistent HR-HPV infection through the expression of host TLR4, NF-κB, C-myc, and hTERT genes, and this effect may be potentiated by sialidase secretion. These findings highlight new opportunities to assess the risk of persistent HR-HPV infection in women of childbearing age.
AUTHOR CONTRIBUTIONS
Binhua Dong and Pengming Sun contributed to the conception and design of the review. Binhua Dong, Yuxuan Huang, Hongning Cai, Yaojia Chen, Ye Li, Huachun Zou, Anping Feng, Heping Zhao, Wenyu Lin, and Hangjing Gao performed the literature review for the manuscript, drafted the manuscript, and created the figures within the manuscript. Huifeng Xue, Yanfang Lu, Xiaodan Mao, Diling Pan, and Zhihui Wu performed the examination of all samples. Pengming Sun provided critical feedback and suggestions to the manuscript. All authors approved the final version of the manuscript.
ACKNOWLEDGMENTS
The authors thank the FCLSC investigators for their contributions to this trial. Above all, we are grateful to all patients who participated in this study. Natural Science Foundation of Fujian Provincial (grant nos. 2017J01232 and 2021J01403); National Key R&D Program of China (grant no. 2021YFC2701205); Fujian Health Young and Middle-aged Scientific Research Major Project (grant no. 2021ZQNZD011); Fujian Provincial Health Commission Innovation Project (grant no. 2019-CX-7); Fujian Provincial Health Education Joint Public Project (grant no. 2019-WJ-05).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
The study was approved by the Ethics Committee of the Fujian Maternity and Child Health Hospital (2020KY015). Written informed consent was obtained from all participants. Informed consent was obtained from all individual participants included in the study.
Open Research
DATA AVAILABILITY STATEMENT
The data not presented in this manuscript have been stored in GenBank. The data that support the findings of this study are available from the corresponding author upon reasonable request.