The complex evolutionary dynamics of SARS-CoV-2, a big challenge to control the pandemic of COVID-19
I found the article published by Magalis et al.1 Magalis et al.1 particularly interesting as the information presented in this article covers two significant aspects that should be considered as a part of the control of the coronavirus disease 2019 (COVID-19) pandemic. The first of these aspects is the potential role of the low-frequency variants in the epidemiology of COVID-19, an issue directly linked with the evolutionary profile of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and the second aspect is the presentation of breakthrough infections among vaccinated individuals, a situation associated with the effectiveness of the current vaccines used during this pandemic.
To date (June 27, 2022), official data by the World Health Organization indicates that the COVID-19 pandemic has produced a total of 539 893 858 cases of COVID-19 resulting in a total of 6 324 112 deaths worldwide (https://covid19.who.int/). These numbers reflect the complexity of this situation in terms of the control of this pandemic and highlights the importance of increasing the knowledge in multiple aspects of the epidemiological triad of SARS-CoV-22, 3
As mentioned above, I believe that one of the main complications in controlling this pandemic is due to the extraordinary and unexpected genome plasticity shown by SARS-CoV-2, a condition reported since the initial stages of this pandemic.4 As a consequence, more than 1300 lineages have been identified based on the Pango lineage designation.5 The genetic relationship among these lineages can be phylogenetically represented by the existence of 14 divergent clades (Figure 1A).6 Furthermore, the rapid evolutionary divergence shown by SARS-CoV-2 during this pandemic, is a phenomenon that may be highly explained by the role of natural selection. In this sense, based on the analysis reported by the adaptive evolution server Datamonkey,7 at least 862 sites in the genome of SARS-CoV-2 have been predicted to be under positive selection. The lack of correlation between the number of sites under positive selection and the length of different genes, indicates that the detection of these sites is not just a mere artifact influenced by the length of different genes (R2 = 0.073, p = 0.221; Figure 1B). Similar results were obtained when validated/predicted cytotoxic T lymphocytes (CTL) epitopes were evaluated8 (Figure 1C).
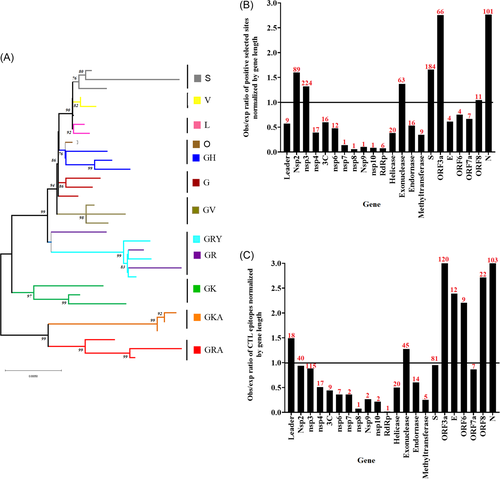
Interestingly, the correlation between the prediction of some positively selected sites and the presence of distinct CTL epitopes (R2 = 0.630, p ≤ 0.0001) suggests that the host immune response may be one of the main drivers in the evolution of SARS-CoV2. In this context, the presence of positively selected sites associated with CTL-escape mutations must be influenced by differences in human leukocyte antigen allele distribution worldwide.
The above data may help to explain the circulation dynamics of different lineages, a condition that can be exemplified by the fluctuation in the proportion of different divergent clades during the pandemic (Figure 2A). Based on the mutation profile in the spike protein, the World Health Organization has classified specific groups of variant lineages as variant being monitored, variant of concern (VOC), or variant of interest. The dominance of these variants has been fluctuating during the pandemic, with the omicron VOC (B.11.529) being the one dominating the infections worldwide during 20229 (Figure 2B).
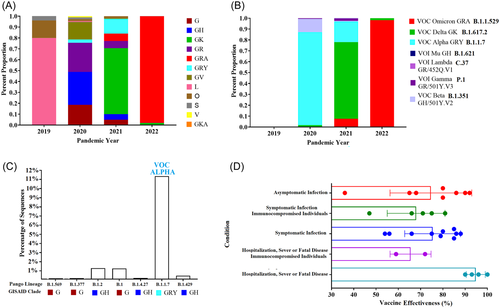
Notably, the work published by Magalis et al.1 presents an interesting perspective that the VOC where not the only variants producing breakthrough infections in vaccinated individuals, but in addition variants of low circulation also played a part in this equation during the pandemic (Figure 2C). The authors report a new set of mutations in the spike protein of lineage B.1.1.7 (L18F and K1191N), which have also come under positive selection during the course of the pandemic (https://observablehq.com/@spond/revised-sars-cov-2-analytics-page). These residues as mentioned previously are predicted/validated to be associated with CTL epitopes.8, 10 Similarly, Magalis et al.1 describes T572I mutation in the spike protein of lineage B.1.2, which also appear to be under positive selection. Future research is needed to understand the role of this mutation.
Finally, this study indirectly covers a relevant issue associated with the effectiveness of RNA vaccines and the presentation of breakthrough infections, a situation that must be considered as a potential issue for the generation of new variants especially in immunocompromised people (Figure 2D).
In conclusion, the work published by Magalis et al.1 reflects the importance of the continuous research on the evolution of SARS-CoV-2, and the necessity to improve current vaccines to promote the control of the COVID 19 pandemic.
ACKNOWLEDGMENTS
The author thanks Dr. Lauren Holinka for her critical review of this letter. This study was conceived in absence of funding.
CONFLICT OF INTEREST
The author declares no conflict of interest
Open Research
DATA AVAILABILITY STATEMENT
No datasets were generated during this study.