Decompensated cirrhosis and liver transplantation negatively impact in DAA treatment response: Real-world experience from HCV-LALREAN cohort
Ezequiel Ridruejo, Federico Piñero, and Manuel Mendizabal have contributed equally to this work as first authors.
Abstract
Introduction
Although the effectiveness of direct-acting antivirals (DAAs) for the treatment of chronic hepatitis C virus (HCV) has been reported in real-world settings, predictive factors of treatment failure are lacking. Therefore, we sought to explore the baseline predictors of treatment response to DAAs.
Methods
This was a prospective multicenter cohort study from the Latin American Liver Research Educational and Awareness Network (LALREAN) including patients who received DAA treatment from May 2016 to April 2019. A multivariate logistic regression model was conducted to identify variables associated with unachieved sustained virological response (SVR), defined as treatment failure (odds ratios [OR] and 95% confidence intervals [CIs]).
Results
From 2167 patients (55.2% with cirrhosis) who initiated DAA therapy, 89.4% completed a full-course treatment (n = 1938). Median treatment duration was 12 weeks, and 50% received ribavirin. Definitive suspension due to intolerance or other causes was observed in only 1.0% cases (n = 20). Overall non-SVR12 was 4.5% (95% CI, 3.5-5.7). There were no significant differences in treatment failure according to HCV genotypes and the degree of fibrosis. Independently associated variables with DAA failure were liver function impairment according to the Child-Pugh score B OR, 2.09 (P = .06), Child-Pugh C OR, 11.7 (P < .0001); and liver transplant (LT) recipient OR, 3.75 (P = .01).
Conclusion
In this real-life setting, higher DAA treatment failure rates were observed in patients with decompensated cirrhosis and in LT recipients. These predictive baseline factors should be addressed to individualize the appropriate time-point of DAA treatment (NCT03775798; www.clinicaltrials.gov).
Highlights
SVR12 failure in clinical practice is low in Latin America (4.5%). End stage liver disease (Child B/C scores) and liver transplantation were independent predictors of DAA failure. Early DAA treatment to prevent disease progression is needed to avoid nonresponse to treatment.
Abbreviations
-
- ASV/DCV
-
- asunaprevir + daclatasvir
-
- CI
-
- confidence interval
-
- CPT
-
- Child-Turcotte Pugh score
-
- CSPH
-
- clinically significant portal hypertension
-
- DAAs
-
- direct-acting antivirals
-
- EBR/GZR
-
- elbasvir/grazoprevir
-
- GLE/PIB
-
- glecaprevir/pibrentasvir
-
- HCC
-
- hepatocellular carcinoma
-
- HCV
-
- hepatitis C virus
-
- HR
-
- hazard ratio
-
- IQR
-
- interquartile range
-
- LDV/SOF
-
- ledipasvir/sofosbuvir
-
- LT
-
- liver transplant
-
- OR
-
- odds ratio
-
- PrOD
-
- paritaprevir/r/ombitasvir/dasabuvir
-
- RAS
-
- resistance-associated substitutions
-
- RWE
-
- real-world evidence
-
- SOF/DCV
-
- sofosbuvir + daclatasvir
-
- SOF/SMV
-
- sofosbuvir + simeprevir
-
- SOF/VEL
-
- sofosbuvir/velpatasvir
-
- SVR
-
- sustained virologic response
-
- VOX
-
- voxilaprevir
1 INTRODUCTION
Hepatitis C virus (HCV) infection is a global health concern with an estimated worldwide disease prevalence of 1%, meaning a value of 71 million infected individuals.1 In America, the estimated prevalence is 0.7%, meaning seven million infected individuals.1 Specifically, in Latin America (LatAm), estimations suggest that only 25% of individuals with suspected HCV infection have been diagnosed, while only 4% has received treatment.2 Access to all oral direct-acting antivirals (DAAs) in the LatAm region is different from Europe and the United States as they have been approved later and some of them are not covered by the health system from each country.
Given the demonstrated efficacy of DAAs in many randomized clinical trials, these treatment regimens have become the HCV standard of care. Advice on their use had been reported in clinical guidelines.3, 4 Also, the effectiveness of DAAs treatment regimens has been reported in many real-world studies around the world. Real-world evidence (RWE) about HCV treatment effectiveness in LatAm is scarce and mostly limited to certain regimens.5-11 Almost all DAAs regimens are approved in most countries in the LatAm region. However, availability among countries may vary and health insurance coverage may be heterogeneous within the region. Access to treatment, given the DAAs high cost, is the main barrier to HCV elimination in almost all LatAm countries although situations vary among them.12
As DDAs' associated sustained virological response (SVR) rate is remarkably high, between 95% and 99%, it is difficult to find treatment response predictors. Genotype 3, bilirubin levels more than 1.5 mg/dL, platelet count less than 120 000/mm3, and the combination sofosbuvir + ribavirin have been associated to DDAs failure in patients with advanced liver disease.13 In another study, also Genotype 3 and male sex were associated to treatment failure.14 Additionally, coinfection with human immunodeficiency virus (HIV) has been associated with reduced SVR rates.15
Our objective was to evaluate predictors of treatment failure to DAAs treatment in a prospective cohort of HCV patients treated with DAAs in a routine clinical practice in LatAm.
2 PATIENTS AND METHODS
2.1 Study design, setting, and participating centers
This prospective cohort study was performed from 1 May 2016 through 30 April 2019 in 23 different Hospitals from Argentina, Brazil, Chile, Colombia, and Uruguay. All eligible patients were enrolled consecutively at each clinical site. Study data were entered into a web-based electronic system. Central revision and resubmission were requested when erroneous or missing data were detected.
Written informed consent was obtained from each patient before enrollment. All study procedures were conducted in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.16 The Austral University Ethics Committee approved this study and each Ethical Committee from all the participating centers approved the study protocol. All procedures followed ethical standards (institutional and national) as well as those mandated by the Helsinki Declaration of 1975, as revised in 2008. The study had a public database for all the investigators on a web link (https://temasis.com.ar/lalrean-org) and received a Research National Grant from the Argentinean National Institute of Cancer. All authors had access to the study data, reviewed and approved the final version of this manuscript. This study was registered in an open public registry (NCT03775798; www.clinicaltrials.gov).
2.2 Cohort characteristics and study variables recorded before DAAs initiation
We included adult patients, older than 18 years old, with chronic HCV infection confirmed by the quantitative real-time polymerase method, and with any degree of liver fibrosis. Genotyping/subgenotyping was conducted before treatment initiation. All patients who received at least one pill of any DAAs were included in the study, as an intention-to-treat analysis.
Baseline exposure variables were recorded for all enrolled subjects including detailed demographic data and relevant past medical history. Evaluation of liver fibrosis stage was assessed by liver biopsy or noninvasive methods (transient elastography or serum biomarkers). Liver stiffness measurements were recorded as a continuous variable in kilopascals (kPa), as obtained from liver elastography and categorized according to international consensus guidelines.17 The cutoffs for advanced fibrosis and cirrhosis were 9.5 and 12.5 kPa, respectively, obtained by transient elastography. Cirrhosis could also be determined by the site investigator or treating physician based on a combination of clinical signs of portal hypertension, biochemical parameters, and/or radiologic findings consistent with cirrhosis.18 The severity of liver disease was evaluated according to the Child-Turcotte Pugh (CTP) score. Decompensated cirrhosis was defined by the CTP B o C and/or a history of ascites, variceal hemorrhage, hepatic encephalopathy, and/or other complications secondary to portal hypertension. Clinically significant portal hypertension (CSPH) was defined as the presence of gastroesophageal varices on endoscopy or presence of a platelet count less than 100 000/mm3 associated with splenomegaly (spleen larger than 120 mm on radiographic imaging).18
2.3 Intervention and main exposure variables
The specific DAAs treatment prescription was based on physicians' criteria according to the available regimens in each country, following national and international guidelines.3, 4 Available DAAs at the time of the study in the LatAm region were asunaprevir + daclatasvir (ASV/DCV), sofosbuvir + daclatasvir (SOF/DCV), sofosbuvir + simeprevir (SOF/SMV), paritaprevir/r/ombitasvir/dasabuvir (PrOD), ledipasvir/sofosbuvir (LDV/SOF), elbasvir/grazoprevir (EBR/GZR), sofosbuvir/velpatasvir (SOF/VEL), and glecaprevir/pibrentasvir (GLE/PIB). All DAA regimens were prescribed according to label and genotypes defined in indications. We assessed the response to DAAs therapy by measuring SVR, defined as an undetectable HCV RNA (HCV viral load <15 UI/mL), 12 weeks after completion of therapy. Laboratory operators were blind to baseline patient characteristics, treatment regimens, and primary outcome events. However, according to study protocol, standard laboratory tests including Model for End-stage Liver Disease (MELD) score and CTP score parameters, as well as assessment of the primary outcome and adverse events, were scheduled at baseline, end-of-treatment, and week 12 posttreatment in all patients.
2.4 Outcome assessment
The primary endpoint analysis was unachieved SVR. Non-SVR or DAAs failure, was defined as a failure to achieve undetectable HCV RNA at 12 weeks after completion or early discontinuation of HCV therapy. Clinical and laboratory assessments were performed at each participant hospital including HCV RNA assays with a lower detection limit of 15 IU/mL.
The secondary objective of the study was a composite end-point including safety profile evaluating adverse events according to Common Terminology Criteria for Adverse Events version 4.03 (CTCAE V4.03), new hepatic decompensation (defined as the development of ascites, hepatic encephalopathy, or variceal bleeding), development of de novo hepatocellular carcinoma (HCC), need for LT or death from any cause during and after treatment completion.19 Safety data were collected from all patients from the time of starting treatment until completion or early discontinuation as per protocol analysis. Serious adverse events (SAEs) including urgent clinic visits, hospitalizations, and/or death were thoroughly reviewed to identify the causal relationship with treatment regimen.
2.5 Statistical analysis
Categorical data were compared using Fisher's exact test (two-tailed) or χ2 test as appropriate. Continuous variables are shown with mean (±standard deviation) or median (interquartile ranges [IQR] 25%-75%) and were compared with the Student t or Mann-Whitney U tests according to their distributions. SVR rates were reported with 95% confidence intervals (CIs). A multiple logistic regression analysis was performed to evaluate baseline exposure variables associated with non-SVR. Unadjusted and adjusted odds ratios (ORs) and its corresponding 95% CI were estimated to evaluate potential confounding effects, defined by a change in the crude OR larger than 20% observed step by step. Variables with a P < .1 after the univariate analysis were included in the multivariable model, generated by stepwise forward selection and comparing each model's performance with the Likelihood ratio test to prioritize a parsimonious model. To avoid overfitting, 1 variable per at least 10 events was included in the multivariate analysis. Final model's calibration and discrimination power was performed using Hosmer-Lemeshow test and receiving operator curve (ROC), respectively. All analyses were performed with STATA 13.0 (StataCorp LLC, College Station, TX).
3 RESULTS
3.1 Population characteristics
From 2167 HCV infected patients prospectively followed and enrolled in LALREAN's registry, 1938 completed 12 weeks posttreatment evaluation and were included in the analysis. The remaining 229 patients were still under treatment, and thus, SVR was not assessed at time of analysis. Baseline characteristics of the study population are shown in Table 1. The mean age of the cohort was 59 (±11) years old, with 55.4% being male. Genotype 1 was the most frequent (72.5%), followed by Genotype 3 (16.9%). Most patients presented with advanced fibrosis (Metavir Score F3-4) (67.9%) and a CPT score A of 81.3% among cirrhotic subjects. Other clinical baseline characteristics regarding HCV infection are shown in Table 2. Regarding treatment history, 1158 (64%) subjects were naive and 780 (36%) were treatment-experienced with at least one IFN-based regimen. Table 3 describes previous treatment history.
| Variables | Values |
|---|---|
| Age, y (mean ± SD) | 59 ± 11 |
| Male gender, n (%) | 1200 (55.4) |
| Median years of known HCV infection, years (IQR) | 7 (2-14) |
| Infection pathway, n (%) | |
| Unknown | 1101 (53.7) |
| Blood transfusion | 402 (19.6) |
| Intravenous drugs use | 262 (12.8) |
| Medical procedure | 162 (7.9) |
| Other | 124 (6.0) |
| HCV genotype, n (%) | |
| 1a | 583 (27.1) |
| 1b | 857 (39.8) |
| 1 (without subgenotype) | 120 (5.6) |
| 2 | 198 (9.2) |
| 3 | 363 (16.9) |
| 4 | 32 (1.5) |
| Other | 14 (0.6) |
| Comorbidities, n (%) | |
| Diabetes mellitus | 321 (14.8) |
| Ischemic heart disease | 66 (3.0) |
| COPD | 31 (1.4) |
| Renal replacement therapy (RRT) | 49 (2.3) |
| Kidney failure without RRT | 18 (0.8) |
| Peripheral vascular disease | 20 (0.9) |
| HIV coinfection, n (%) | 243 (11.2) |
| HBV coinfection, n (%) | 12 (0.5) |
| Liver transplant recipient, n (%) | 151 (6.7) |
| Kidney transplant recipient, n (%) | 73 (3.3) |
- Abbreviations: COPD, chronic obstructive pulmonary disease; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IQR, interquartile range 25% to 75%; RRT, renal replacement therapy with dialysis; SD, standard deviation.
| Variables | Values |
|---|---|
| Liver involvement | |
| Fibrosis grade by invasive or noninvasive methods, n (%) | |
| F0 | 84 (3.9) |
| F1 | 431 (19.9) |
| F2 | 180 (8.3) |
| F3 | 275 (12.7) |
| F4 | 1197 (55.2) |
| Cirrhosis, n (%) | 1197 (55.2) |
| History of decompensated cirrhosis, n (%) | 313 (14.4) |
| Ascites | 199 (9.5) |
| Hepatic encephalopathy | 84 (3.9) |
| Variceal bleeding | 91 (4.2) |
| HCC | 62 (2.9) |
| CSPH, n (%)* | 800 (36.9) |
| Child-Pugh A/B/C, (%) | 973 (81.3)/201 (16.8)/23 (1.9) |
| MELD score (mean ± SD) | 8.9 ± 3.2 |
| Extrahepatic manifestations | |
| Mixed cryoglobulinemia, n (%) | 66 (3.0) |
| Glomerular disease, n (%) | 13 (0.6) |
| Non-Hodgkin lymphoma, n (%) | 18 (0.8) |
| Porphyria cutanea tarda, n (%) | 31 (1.4) |
| Lichen planus, n (%) | 6 (0.3) |
| Other manifestations, n (%) | 76 (3.5) |
- Note: *CSH defined as presence of at least one of the following: ascites, gastroesophageal varices or hepatic encephalopathy or splenomegaly and platelet count less than 100 000/mm3.
- Abbreviations: CSH, clinically significant portal hypertension; HCC, hepatocellular carcinoma.
| Variables | Values |
|---|---|
| Previous HCV treatment, n (%) | 780 (36.0) |
| Previous interferon-based HCV treatment, n (%) | |
| INF + RBV | 167 (7.7) |
| PegINF + RBV | 550 (25.4) |
| Previous triple therapy PegINF/RBV, n (%) | |
| Boceprevir | 71 (3.3) |
| Telaprevir | 69 (3.2) |
| Previous DAAs failure, n (%) | 9 (0.4) |
| Sofosbuvir-based regimen | 7 |
- Abbreviations: DAAs, direct antiviral agents; HCV, hepatitis C virus; INF, interferon α; PegINF, pegylated INF; RBV, ribavirin.
The most frequently used DAAs regimen was SOF/DCV, being applied in 68.3% patients (n = 1480), followed by PrOD in 12% (n = 260), and LDV/SOF in 6.1% (n = 132). Overall median treatment duration was 12 weeks (IQR 11.8-15.3 weeks). Ribavirin was prescribed in 809 (37.3%) patients (Table 4). Median treatment duration in noncirrhotic and cirrhotic patients was 12.0 weeks (IQR 11.8-12.3 weeks) and 12.6 weeks (IQR 12.0-23.8 weeks) (P < .0001), respectively. According to genotypes, median treatment duration was 12 weeks (IQR 11.8-17.0 weeks) in Genotype 1, 12 weeks (IQR 11.8-14.0 weeks) in Genotype 1a, 12 weeks (IQR 11.8-23.8 weeks) in Genotype 1b, 12 weeks (IQR 12.0-13 weeks) in Genotype 2, 12 weeks (IQR 11.8-24 weeks) in Genotype 3, and 12 weeks (IQR 11.8-13.8 weeks) in Genotype 4.
| Variables | Values |
|---|---|
| Started treatment, n (%) | 2167 (100) |
| Completed treatment, n (%) | 1938 (89.4) |
| Median pretreatment HCV viral load, IU/mL (IQR) | 1 192 000 |
| (369 000-3 600 000) | |
| Median treatment duration, weeks (IQR) | 12.0 (11.8-15.3) |
| 12 wk-treatment, n (%) | 1032 (53.2) |
| 24 wk-treatment, n (%) | 906 (46.7) |
| Definitive treatment discontinuation, n (%) | 26 (1.5) |
| Intolerance | 1 (0.05) |
| Loss of follow-up | 18 (0.8) |
| Access | 4 (0.2) |
| Median follow-up since DAAs treatment, months (IQR) | 22.7 (13.2-32.8) |
| Use of ribavirin, n (%) | 809 (37.3) |
| Median ribavirin dose, mg (IQR) | 1000 (800-1000) |
| Sofosbuvir, n (%) | 1595 (73.6) |
| Non-generic | 903 (41.7) |
| Generic | 689 (31.8) |
| Ledipasvir/Sofosbuvir, n (%) | 132 (6.1) |
| Sofosbuvir + Daclatasvir, n (%) | 1480 (68.3) |
| Sofosbuvir/Velpatasvir, n (%) | 51 (2.3) |
| Sofosbuvir + Simeprevir, n (%) | 6 (0.3) |
| Daclatasvir/Asunaprevir, n (%) | 25 (1.1) |
| Daclatasvir + Simeprevir, n (%) | 3 (0.1) |
| Paritaprevir/Ombitasvir/ritonavir/Dasabuvir, n (%) | 260 (12.0) |
| Paritaprevir/Ombitasvir/ritonavir (GT4 only), n (%) | 4 (0.2) |
| Grazoprevir/Elbasvir, n (%) | 83 (3.8) |
| Glecaprevir/Pibrentasvir, n (%) | 12 (0.5) |
- Abbreviations: DAAs, direct antiviral agents; HCV, hepatitis C virus; IQR, interquartile range 25% to 75%.
Overall, the SVR rate was 95.1% (95% CI, 93.9-96.1). According to fibrosis stages, the SVR rate was 95.7% (95% CI, 94.4-96.8) in F0-2% and 93.6% (95% CI, 91.1-95.5) in F3-4 patients. SVR rates were not significantly different among genotypes (P = .80) and fibrosis stages (P = .09) (Figure 1). Stratified SVR rates according to liver disease severity (noncirrhotic vs cirrhotic patients) are shown in Table 5. In noncirrhotic subjects' median treatment duration was shorter (12 weeks [IQR 11.8-12.3] vs 12.6 weeks [IQR 12-23.8]; P < .0001) and fewer patients required ribavirin (22.5% vs 49.4%; P < .0001). Regarding clinical outcomes, there was a lower rate of adverse events (20.0% [95% CI, 17.5-22.6] vs 29.8% [95% CI, 27.2-32.5]; P < .0001) and similar SVR rates (94.7% [95% CI, 92.8-96.2] vs 95.4 [95% CI, 93.9-96.6]; P = .51) when comparing noncirrhotic and cirrhotic patient groups, respectively.
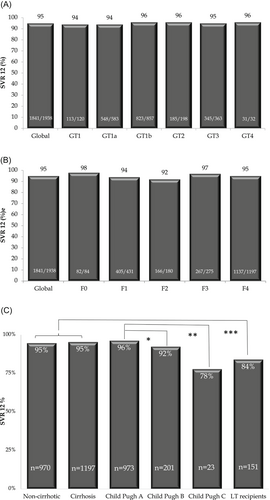
| Variables | Noncirrhotic (n = 970) | Cirrhotic (n = 1197) | P |
|---|---|---|---|
| Completed treatment, n (%) | 835 (86.1) | 1103 (92.1) | |
| Median HCV viral load, UI/mL (IQR) | |||
| Median treatment duration, weeks (IQR) | 12 (11.8-12.3) | 12.6 (12-23.8) | <0.0001 |
| 12 wk-treatment, n (%) | 576 (69.0) | 456 (41.3) | <0.0001 |
| 24 wk-treatment, n (%) | 259 (31.0) | 647 (58.7) | |
| Treatment discontinuation, n (%) | |||
| Intolerance | 10 (1.3) | 16 (1.6) | 0.52 |
| Loss of follow-up | 30 (3.9) | 31 (3.0) | 0.52 |
| Median follow-up since DAAs, months (IQR) | 19.2 (10.5-29.3) | 25.4 (15.6-35.4) | <0.0001 |
| Ribavirin, n (%) | 218 (22.5) | 591 (49.4) | <0.0001 |
| Sofosbuvir, n (%) | 699 (72.1) | 896 (74.8) | 0.14 |
| Sofosbuvir-Daclatasvir, n (%) | 651 (67.1) | 829 (69.3) | 0.28 |
| Paritaprevir/Ombitasvir/Dasabuvir, n (%) | 115 (11.9) | 145 (12.1) | 0.85 |
| Sofosbuvir-Ledipasvir, n (%) | 69 (7.1) | 63 (5.3) | 0.07 |
| Grazoprevir/Elbasvir, n (%) | 50 (5.1) | 33 (2.8) | 0.004 |
| Sofosbuvir-Velpatasvir, n (%) | 29 (3.0) | 22 (1.8) | 0.08 |
| Glecaprevir/Pibrentasvir, n (%) | 10 (1.0) | 2 (0.2) | 0.007 |
| SVR12, % (95% CI) | 94.7 (92.8-96.2) | 95.4 (93.9-96.6) | 0.51 |
| Any AE, % (95% CI) | 20.0 (17.5-22.6) | 29.8 (27.2-32.5) | <0.0001 |
| Death, n (%) | 4 (0.4) | 12 (1.0) | 0.11 |
| Liver decompensation, n (%) | 14 (1.4) | 13 (1.1) | 0.45 |
| HCC, n (%) | 18 (1.9) | 25 (2.1) | 0.70 |
- Abbreviations: AE, adverse event; DAAs, direct antiviral agents; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; SVR, sustained virological response.
3.2 Baseline variables independently associated to DAAs failure
In univariate analysis, pretreatment variables associated with DAAs failure were male gender, LT, previous IFN-based treatment, decompensated cirrhosis (CTP B or C vs A), CSPH, and platelet count less than 100 000 mm3. Independently associated variables with DAAs failure were liver function impairment according to the Child-Pugh score B OR, 2.09 (95% CI, 0.95-4.61; P = .06) and Child-Pugh C OR, 11.7 (95% CI, 3.15-43.58; P < .0001) vs Child A as reference value; decompensated cirrhosis OR, 2.40 (95% CI, 1.33-4.35; P < .0001) vs nondecompensated cirrhosis as reference value; and LT recipient OR, 3.75 (95% CI, 1.37-10.28; P = .01) (Table 6). Baseline characteristics of decompensated cirrhosis and LT patients are shown in Tables S1 and S2. There were some differences in treatment regimens according to current treatment guidelines for these special populations.
| Variables | non-SVR12 (%) | Unadjusted OR (95% CI) | P | Adjusted OR (95% CI) | P |
|---|---|---|---|---|---|
| Age, y | 1.00 (0.98; 1.02) | .74 | |||
| Gender | |||||
| Male (n = 1200) | 6.0 | 1.78 (1.12; 2.81) | .014 | 1.78 (0.89; 3.54) | .10 |
| Female (n = 967) | 3.5 | ||||
| Diabetes mellitus | |||||
| Yes (n = 321) | 5.8 | 1.25 (0.71; 2.19) | .43 | ||
| No (n = 1846) | 4.7 | ||||
| HIV coinfection | |||||
| Yes (n = 243) | 6.1 | 1.31 (0.69; 2.45) | .40 | ||
| No (n = 1924) | 4.7 | ||||
| HBV coinfection | |||||
| Yes (n = 12) | 0 | - | |||
| No (n = 2155) | 4.9 | ||||
| Liver transplant | |||||
| Yes (n = 151) | 15.6 | 4.28 (2.45; 7.48) | <.0001 | 3.75 (1.37; 10.28) | .01 |
| No (n = 2016) | 4.1 | ||||
| Kidney transplant | |||||
| Yes (n = 73) | 9.4 | 2.10 (0.81; 5.38) | .13 | ||
| No (n = 2094) | 4.8 | ||||
| Previous INF-based regimens | |||||
| Yes (n = 780) | 6.5 | 1.76 (1.14; 2.72) | .01 | 1.11 (0.58; 2.12) | .74 |
| No (n = 1387) | 3.8 | ||||
| Ribavirin use | 1.35 (0.88; 2.08) | .17 | |||
| Yes (n = 809) | 5.7 | ||||
| No (n = 1358) | 4.3 | ||||
| Fibrosis grade | |||||
| F0 (n = 84) | 1.8 | ||||
| F1 (n = 431) | 6.4 | 3.63 (0.47; 27.7) | .21 | ||
| F2 (n = 180) | 8.2 | 4.97 (0.63; 39.2) | .13 | ||
| F3 (n = 275) | 2.9 | 1.74 (0.21; 14.4) | .61 | ||
| F4 (n = 1197) | 4.6 | 2.70 (0.36; 19.9) | .33 | ||
| LSM, kPa | |||||
| ≤23 (n = 1005) | 5.2 | 1.39 (0.64; 3.05) | 0.40 | ||
| >23 (n = 136) | 4.4 | ||||
| Child-Pugh | |||||
| A (n = 973) | 3.7 | … | … | … | |
| B (n = 201) | 7.6 | 2.15 (1.08; 4.30) | .029 | 2.09 (0.95; 4.61) | .06 |
| C (n = 23) | 22.2 | 7.50 (2.33; 24.1) | .001 | 11.7 (3.15; 43.58) | <.0001 |
| CSPH* | |||||
| Yes (n = 800) | 7.5 | 3.38 (2.18; 5.24) | <.0001 | 0.83 (0.37; 1.88) | .67 |
| No (n = 1367) | 2.3 | ||||
| Albumin gr/dL | |||||
| <3.5 (n = 207) | 5.3 | 0.69 (0.29; 1.64) | .41 | ||
| ≥3.5 (n = 1421) | 4.6 | ||||
| Platelets < 100 000/mm3 | |||||
| Yes (n = 347) | 5.8 | 2.31 (1.40; 3.78) | .001 | 1.77 (0.79; 4.00) | .17 |
| No (n = 1425) | 3.6 | ||||
| AFP > 20 ng/mL | |||||
| Yes (n = 90) | 5.6 | 1.95 (0.86; 4.35) | .11 | ||
| No (n = 731) | 3.5 |
- Note: *CSPH defined as presence of at least one of the following: ascites, gastroesophageal varices or hepatic encephalopathy or splenomegaly and platelet count less than 100 000/mm3. Overall non-SVR was 4.9% (95% CI, 3.9-6.0%). Hosmer-Lemeshow test P = .88, calibration between expected and observed events.
- Abbreviations: CSPH, clinically significant portal hypertension; CI, confidence interval; HBV, hepatitis B virus; HIV, human immunodeficiency virus; INF, interferon α; LSM, liver stiffness measurement; OR, odds ratios; SVR, sustained virological response.
3.3 Safety
During a median follow-up period of 22.3 months (IQR 12.6-32.2 months), 91 patients presented evidence of clinical disease progression, with a cumulative incidence of 4.2% (95% CI, 3.4-5.2%). From them, 16 patients died, 66 patients presented a new liver decompensation event, 8 patients underwent LT, and 41 patients developed de novo HCC.
During treatment, any adverse event was reported in 25.4% (95% CI, 23.5-27.3%) of patients. The main adverse events were asthenia 15% (n = 325), headache 5.6% (n = 122), vomiting 3.1% (n = 67), insomnia 3.0% (n = 65), diarrhea 2.4% (n = 52), arthralgia or myalgia 1.5% (n = 32), rash 1.1% (n = 25), increased total bilirubin levels in 1.1% (n = 23), and fever 0.5% (n = 12). Definitive treatment discontinuation was observed in 1.5% of patients (n = 26) due to intolerance in one patient, access barriers in 4 patients and loss of follow-up in 18 patients.
4 DISCUSSION
Results of this large prospective cohort study from the LALREAN registry shows a low failure rate to achieve SVR: 4.9% (95% CI, 3.9-6.1), not describing any significant differences among genotypes and fibrosis stages. Among all the baseline variables included in the adjusted analysis, only liver function impairment according to the Child-Pugh score B and C and LT recipient were independently associated with failure to achieve SVR.
Overall, our results are similar to those recently reported by smaller studies conducted in the LatAm region analyzing only one DAA regimen.5-11 In the first report of the LALREAN registry, including 900 patients from Brazil and Argentina, the overall SVR12 rate with SOF/DCV ± RBV was 96.1% (95% CI, 94.6-97.2%). LT recipients and CTP B and C were also independently associated with failure to achieve SVR.5 In a study from Brazil, 3939 patients treated with SOF-based DAAs regimens achieved a 95% SVR rate. Again, cirrhosis and previous nonresponders were associated with lower chances of achieving SVR.11 In addition, our results resemble those obtained in large RWE cohorts from Europe and North America and from randomized clinical trials. In the European studies, baseline factors associated with higher DAA failure rates included Genotype 3, NS5A resistant-associated substitutions (RAS), bilirubin levels more than 1.5 mg/dL, platelet count less than 120 000/mm3, advanced fibrosis (Metavir F3-F4), HIV coinfection, and previous IFN-based treatment.13, 14, 20, 21 In the United States studies, Genotype 3, NS5A-RASs, decompensated cirrhosis, and previous DAAs failure were significantly associated with a reduced SVR rate.22-24 The reasons for this reduced SVR rate in patients with advanced fibrosis and LT are not clearly understood but may be multifactorial. Potential reasons are altered pharmacokinetics of drug uptake, distribution, and metabolism; immune derangements such as dampened chemoattraction of T lymphocytes and interaction of T lymphocytes and HCV-infected hepatocytes, and lymphopenia and low CD4 T helper cell counts due to splenomegaly.25, 26 It may also be related to intolerance (higher with RBV use), nonadherence, significant comorbidities, drug-drug interactions, and others26
Patients with active HCC were not included in our cohort so we cannot evaluate if it was associated with failure to achieve SVR. However, in a cohort from Taiwan, 1.3% of patients without HCC, 2.9% of patients with inactive HCC, and 13.0% patients with active HCC did not achieve SVR.27 In the multivariate analysis, active HCC (vs inactive HCC and non-HCC) was associated with DAAs failure (OR, 24.5 [95% CI, 4.4-136.9], P < .001).27 Two recently published meta-analysis confirmed a reduced SVR rate in patients with active HCC when compared with patients with inactive HCC or without HCC.28, 29
Our results are similar to those previously reported by other cohorts describing advanced liver disease as one of the main predictors of DAAs failure. Even with highly effective DAAs, it can still impact negatively on SVR rates. This emphasizes the need of treating patients at earlier stages of liver disease to avoid disease progression, particularly in regions like LatAm where access to treatment in the early stages of liver diseases is restricted. In a previous study from our group we reported improvements in clinical outcomes in patients achieving SVR, and with a longer follow up, we expect to evaluate other benefits such as improvement in fibrosis.30 Nonetheless, patients with advanced liver fibrosis who achieved SVR are still at risk, albeit low, of developing liver-related complications.30, 31 Treating patients at milder stages of liver disease results in higher SVR rates, reduced risk of liver disease progression, and extrahepatic HCV-related events.32 To pursue WHO's ambitious goal of eliminating viral hepatitis as a public threat by 2030, we must overcome many barriers to treatment access in LatAm.12, 33 DAA failures can be reduced with the use on the newer and more potent pangenotypic regimens such as SOF/VEL, SOF/VEL/VOX, and GLE/PIB; these third-generation DAAs reach 98% to 99% SVR rates.3, 4 RWE studies conducted with these DAAs showed 99% SVR rates, the same results obtained in randomized clinical trials. These SVR rates were not influenced by gender, previous treatment, treatment duration, fibrosis, or chronic kidney disease stage.34-36 Although these DAAs are available in some countries in the LatAm region, access is restricted making their prescription difficult for the clinicians. Therefore, we still depend on older and less potent DAAs, making early treatment the best strategy to reduce DAAs failure.
A significant strength of our study is the prospective and multicenter design that allowed us to analyze well-documented variables associated with DAA failure. However, we faced some limitations. First, in our cohort, the most common regimen used was SOF/DCV, which is no longer used in other regions. Nevertheless, our findings reflect the reality in low and middle-income countries with economic limitations to access newer pangenotypic regimens. Second, baseline RAS was not evaluated given that its use is limited in our region. Finally, patients with more advanced liver disease and a higher risk of DAA failure might not be selected for treatment.
In summary, our study demonstrates that patients with advanced liver disease or those who underwent liver transplantation present a higher rate of DAA failure. It is essential to apply a new strategy in LatAm and treat all individuals with HCV, irrespective of their disease stage, to decrease not only morbidity and mortality but transmission, as well. To achieve this goal, we need the development of public health programs adapted to each national setting.
ACKNOWLEDGMENTS
To Silvina Heisecke, from CEMIC-CONICET, for the copyediting of the manuscript. This study has received financial support from the Argentinean National Institute of Cancer and an unrestricted research grant from Abbvie, Merck Sharp Dome, and Bristol-Myers Squibb.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
Guarantor of the article: ER. Specific author contributions: ER, FP, MM, and MS designed the research study, performed the research, collected, and analyzed the data, and wrote the paper. All authors performed the research, collected the data, and co-wrote the paper. All authors approved the final version of the manuscript.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.