Kawasaki disease seasonality in Venezuela supports an arbovirus infection trigger
Abstract
Kawasaki disease (KD) is an inflammatory disease primarily affecting infants and young children, whose etiology remains uncertain. Observational studies of the overlap between KD outbreaks and seasonal peaks of arboviral infections, suggest the possible role of these pathogens as triggers of KD. In Venezuela, regions with the highest reported arboviral infections simultaneously have the highest incidence of KD. One proposed explanation for this association involves the role of proinflammatory mediators, interleukin-1 (IL-1), IL-6, tumor necrosis factor, and vascular endothelial growth factor as mediators of coronary endothelial damage. The promotion of inflammation and tissue destruction by these cytokines is thought to contribute to the coronary endothelial damage experienced in KD. The utilization of overlapping KD and arboviral infection trends contribute to the comprehension of KD etiology, with improvements in diagnosis, prognosis and treatment.
Highlights
-
Kawasaki disease is an inflammatory disease primarily affecting infants and young children.
-
Overlap between Kawasaki disease outbreaks and seasonal peaks of arboviral infections suggest a role for these pathogens as potential triggers for the disease.
-
IL-1 has been linked to tissue injury and vascular leakage in Dengue, and also has a key role in Kawasaki disease pathogenesis.
-
Arbovirus modulation of the immune response may be influenced by viral kinetics, host-related susceptibility and specific cytokine signatures that may contribute to disease severity.
1 INTRODUCTION
Kawasaki disease (KD), or mucocutaneous lymph node syndrome, is an acute, idiopathic multisystem febrile vasculitis that primarily affects infants and young children,1 and is the most common cause of acquired cardiac disease among that age group in industrialized countries. Although usually self-limited, untreated KD may evolve into a life-threatening condition with increased risk of coronary artery vasculitis.1
These clinical features, in combination with the observation of KD outbreaks and seasonal peaks over the endemic background, support an infectious, possibly viral, trigger for KD. Herein we discuss a potential role for arthropod borne viruses (arboviruses) as triggers for KD in tropical, subtropical, and temperate regions, based on recent experience from Venezuela and global epidemiologic trends in seasonality of arbovirus infections and observations of KD.
2 EPIDEMIOLOGIC TRENDS IN VENEZEULA AND COMPARISON WITH OTHER REGIONS
In Venezuela, KD cases are diagnosed year-round, but with a bimodal peak incidence in the months of March and November, coinciding in many regions with the rainy seasons.2, 3 These bimodal peaks resemble the two seasonal peaks observed in temperate South-East Asian countries (December-January/June-July) (Table 1). Epidemiologic trends in Venezuela for KD and arboviral infections suggest a potential link between these illnesses. As arboviral outbreaks have increased in frequency and magnitude, a parallel increase in KD has followed, with similarities in clustering of cases and geographic wavelike spread.
| Virus | Vector | Region | Virus seasonality | KD seasonality |
|---|---|---|---|---|
| DENV | Mosquito (Aedes sp.) | Americas. | Mexico (late summer-early fall).4 | Year-round, peaks following rainy season. |
| Brazil (January to April, with a peak in March-April).4 | Brazil: February to May.5 | |||
| Venezuela (March to November, with a peak between August and November).6 | Venezuela: March and November.2 | |||
| Western Pacific (Hawaii). | Year-round. | Year-round.7 | ||
| South-east Asia. | Year-round; Thailand Indonesia. | Year-round.5 | ||
| JEV | Mosquito (Culex sp.) | Tropical south-east Asia/Western Pacific. | Year-round, with a peak during rainy season.8, 9 | Year-round.10 |
| WNV | United States (temperate states). | Late summer/early fall.11 | Winter months, peak in summer months. | |
| San Diego: Winter (January-March)/spring peak, summer peak (June).10, 12 | ||||
| United States (southern states). | Year-round.11 | Insufficient data available to determine seasonality. | ||
| YFV | Mosquito (Aedes sp.) | Sub-Saharan Africa. | September-October, spring/early summer; end of rainy season, beginning of dry season.13 | Insufficient data available to determine seasonality. |
| Mosquito (Haemagogus sp.) | South America. | January-May with a peak in February and March (rainy season).11 | Year-round, peaks following rainy season. |
- Abbreviations: DENV, dengue virus; KD, Kawasaki disease; JEV, Japanese encephalitis virus; WNV, Western Nile virus; YFV, yellow fever virus.
During the period from 1985 to 2011, reports of KD in Venezuela have increased steadily.2 The highest incidence occurred in years 2005, 2009, and 2010,2 coinciding with Venezuela's interannual and seasonal cycles of dengue virus (DENV) epidemics (1-4 years; P < .05) (Figure 1A).6 The rapid wavelike spread of KD cases observed in 2009 to 2010 overlapped with the largest dengue epidemic ever recorded in Venezuela.6 Indeed, almost one of every five KD cases reported in Venezuela has been clinically associated with concurrent DENV infection,2 thus suggesting a significant link between KD cases and the periodic outbreaks of this arbovirus. Notably, the north and central western regions of Venezuela, which have reported the most KD cases, also have been the areas most affected by arbovirus spread.14 In fact, the higher incidence of KD cases reported in the states of Lara, Zulia, Mérida, Táchira, Carabobo, Aragua, and Capital District mirrors the regional dengue incidence; the higher population density areas (central regions) and the regions bordering Colombia (border regions) exhibit a higher DENV incidence14 and also KD incidence2, 3 (Figure 1B). Furthermore, during the past decade, there have been explosive epidemics in Venezuela due to other arboviruses, most notably chikungunya virus (CHIKV) and zika (ZIKV), which now co-circulate, and share a broad range of dengue-like symptoms and potential complications.16 Venezuela has been severely affected by both CHIKV and ZIKV, which have spread rapidly through the same densely populated regions that have high endemic DENV transmission.14 The temporal association between the incidence of new KD cases, and the surge in DENV, CHIKV, and ZIKV cases is consistent with a potential role for these viruses in the development of KD.
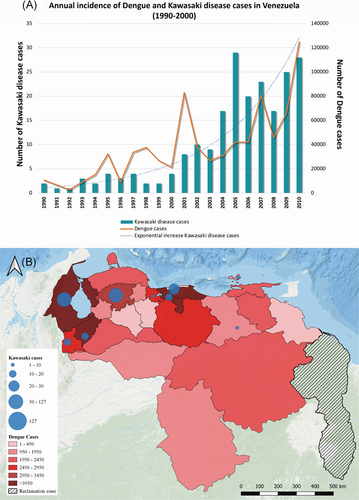
Although DENV, CHIKV, and ZIKV demonstrate the most compelling evidence in their relationship with the seasonal development of KD in Venezuela, there are periodic cases of KD cases, which remain without correlation. These outliers may be due to other circulating, and neglected, arboviruses of the Togaviridae family in Venezuela, including Mayaro virus (MAYV) and Venezuelan equine encephalitis virus (VEEV). MAYV is a recent emerging arbovirus, which demonstrates many similarities in clinical syndrome with CHIKV, DENV, and ZIKV.17 VEE is a neglected tropical disease with both epizoonotic and enzoonotic subtypes, which is thought to contribute to KD through focal infectious outbreaks occurring periodically.18
A similar relationship can be observed in infections with Japanese encephalitis virus (JEV), a Culex mosquito-transmitted flavivirus endemic in South-East Asia and the Western Pacific.8, 9 JEV transmission mainly infects children and most often occurs in rural, rice-cultivating communities where flood irrigation is practiced, but can also present in urban areas.8 In temperate areas of Asia, including Japan, China, Taiwan, and the Korean peninsula, JEV infection peaks in the summer months, following the rainy season.8, 9 Similar seasonal trends with peaks in KD occurrence following months of copious rainfall, have been reported from Japan and Korea.10, 19
Notably, in more tropical regions of Asia, such as Cambodia, Indonesia, southern Vietnam, and southern Thailand, in which JEV transmission occurs year-round.20 In correlation, KD cases also present year-round, without any distinguishable seasonal variations.5, 21
In Hawaii, which leads the United States in reported KD, a parallel can be drawn between the occurrence of KD cases year-round, hypothesized to be a result of the tropical climate,7 and Hawaii's year-round mosquito season with its corresponding increased risk of DENV infection.22 In contrast, studies in Southern California, specifically San Diego, suggested a seasonal trend in KD cases resembling that in Japan, with seasonal peaks in the winter/spring months (January-March) and a lesser peak in June, although no definitive viral association has yet been identified.5, 12
The seasonality of viral transmission and reports of KD cases in different regions are compared in Table 1 for different arboviruses.
Defining a precise temporal relationship between arboviral infection peaks and KD development has been hampered by the reliance on voluntary reporting for surveillance of KD cases in many locales. Review of case reports in the literature support time frames between 3 and 11 days from fever development due to DENV infection and clinical presentation of KD, including rash, cracked lips, organomegaly, conjunctivitis, etc.23-25 One intrinsic limitation is that many reports of KD in association with arboviral infection (DENV, CHIIK, and ZIKV) assessed patient presentation retrospectively, making the true temporal relationship difficult to assess.
3 ARBOVIRAL INFECTION AS AN UNDERLYING CAUSE OF KAWASAKI DISEASE
Although the underlying cause of KD remains elusive, links have been established through serological and nucleic acid amplification analyses of clinical samples to numerous viral agents. Hypothesized viral associations include adenovirus, Epstein-Barr virus, enterovirus, human bocavirus, human coronaviruses, human herpesvirus 6, human immunodeficiency virus, human metapneumovirus, influenza viruses A and B, measles, parainfluenza virus types 1, 2, and 3, rhinovirus, RSV, parvovirus B19, and Varicella-Zoster virus. KD outbreaks in the United States have been linked to preceding viral illnesses12, 23-25 associated with respiratory26 and gastrointestinal symptoms.26, 27 Several arthropod-borne RNA viruses (arboviruses) have also been incriminated as possible triggers of KD. Of note, RNA virus-like inclusion bodies have been found in the cytoplasm of broncho-epithelial cells from patients with KD, in conjunction with the presence of immunoglobulin A-producing plasma cells in affected arterial tissues.28
Arboviruses, which include members of the Flaviviridae (genus Flavivirus), Togaviridae (genus Alphavirus), Bunyaviridae (Bunyavirus, Orthobunyavirus, Nairovirus and Phlebovirus genera), and some members of the Reoviridae, Rhabdoviridae, and Orthomyxoviridae families,29 are widely distributed across all seven continents. This global distribution, in conjunction with the high mutational frequency of the arbovirus RNA genome, allows rapid adaptation to alternating host (vertebrate and invertebrate) and environmental conditions.29 Consequently, arboviruses have exceptional potential for vector adaptation and expansion, including rapid emergence following anthropogenic movements.29 Other major drivers that can affect arbovirus distribution and expansion include climatic changes, and a transmission cycle that includes low level endemic virus transmission within wild animals (enzootic), higher level epidemic transmission affecting peridomestic/domestic animals (epizootic), and epidemic cycles in humans.29, 30 Continuing urbanization further facilitates the emergence of arboviruses due to niche transgression, and provides enhanced opportunities for contact between anthropophilic vectors and susceptible human hosts, setting the stage for virus amplification.30 Notably, a similar association between urbanization and KD incidence has been reported.31
Furthermore, several arboviruses, particularly DENV and CHIKV32 and more recently ZIKV33 have been identified as causative of human viral myocarditis, although the mechanisms leading to their cardio-virulence remain to be deciphered. Moreover, the togavirus CHIKV, and flaviviridae ZIKV and DENV, have been shown to exhibit specific tropism for endothelial cells,34 suggesting an important role as potential players in virus-induced post-infectious vasculitis.
4 POTENTIAL MECHANISM: A ROLE FOR INTERLEUKIN-1, INTERLEUKIN-6, AND OTHER CYTOKINES
The potential etiologic mechanisms suggested for KD include autoantigen/autoimmune phenomena, superantigen response, and direct infectious vasculitic injury.35 In addition, there is mounting evidence that KD may be associated with a dysregulated immune response against a variety of pathogens,36 and that certain genetic backgrounds may confer susceptibility to KD development after exposure to potential infectious triggers.37
Shortly after entry following a mosquito bite, most arboviruses will invade resident cutaneous cells, such as keratinocytes, which are permissive to viral replication during early stages of infection.38 This initial interaction between virus and host resident cells triggers a series of sensing, apoptosis, and signaling pathways, as part of the innate immune response.36, 37 Responses to arboviral infection include activation and production of type 1 interferons (IFN) via specific induction of pattern recognition receptors, and upregulation of expression of IFN-stimulated genes and cytokines, such as interleukin-1 (IL-1), IL-6, and tumor necrosis factor (TNF) that promote T-cell attraction and direct receptor independent-like antiviral responses36, 38-40 (Figure 2A).
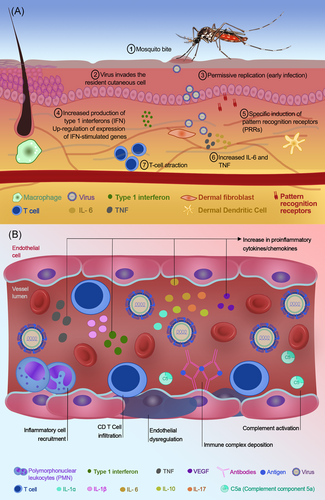
In the context of arboviral infection, coronary endothelial damage experienced by patients with KD may be associated with upregulated production of pro-inflammatory cytokines/chemokines, such as IL-1, IL-6, TNF, and vascular endothelial growth factor, which promote inflammation and tissue destruction. Levels of IL-6, IFNγ, and TNF-α have been reported to be significantly increased in KD affected patients and to decrease after IVIG treatment.41 High levels of IL-6, IL-10, and IFNγ have been correlated to an increased risk for development of coronary artery lesions41 (Figure 2B).
IL-1β, which has been linked to induce tissue injury and vascular leakage in DENV infection, also has a key role in KD pathogenesis. Both IL-1α and IL-1β are essential players in the immunopathogenesis of KD associated vasculitis and coronary arteritis42 by promoting a proinflammatory milieu leading to chemotaxis, tissue damage, and vascular dilation.40 Recent trials supporting the effectiveness of IL-1 blockade treatment in KD patients have provided further evidence for the importance of IL-1 signaling in KD pathogenesis.40
IL-6 and TNF-α are known to be significantly increased in patients with DENV when compared with healthy controls, while IFNγ levels correlate with disease severity.43 Furthermore, severely-affected patients with CHIKV exhibit higher IL-6 levels than CHIKV mildly-affected patients in addition to increased IFN-γ and high level of C5a as a result of complement activation.44 Complement activation may contribute to inflammatory vascular damage by promoting recruitment of inflammatory cells and through direct injury to endothelial cells.36 An increase in proinflammatory cytokines (IL-1β, IL-6, TNF-α, IFN-γ, and IL-17) has also been reported to occur in ZIKV infected patients presenting with cardiac complications.45 This shared proinflammatory cytokine signature observed in KD and amongst different arboviruses suggests that arboviral infections are likely to contribute to KD pathogenesis.
In addition, Brown et al46 demonstrated infiltration by CD45RO+ (activated or memory) T cells and cytotoxic CD8+ T cells in coronary artery aneurysms from patients with fatal acute KD coronary arteritis, concluding this finding supported the presence of an intraepithelial pathogen, most likely viral, causing endothelial injury.46
In dengue shock syndrome, multiple mechanisms have been implicated in disruption of the vascular endothelial barrier. T-cell mediated reactions, presence of cross-reacting antibodies within the vascular endothelium, complement activation, and enhancing antibodies and immune complex deposition,47 have been proposed to contribute to endothelial dysregulation and increased vascular disruption and permeability. Recent findings also highlight a potential role for the flavivirus nonstructural protein 1 in arboviral-induced endothelial dysfunction.34 For example, Japanese encephalitis, West Nile, Yellow fever, and ZIKV selectively bind to human endothelial cells, disrupting different endothelial glycocalyx components and leading to endothelial hyperpermeability.34
Arbovirus modulation of the immune response may be influenced by viral strain-related replication kinetics, as seen in cases of VEEV infection,48 or by host- related susceptibility factors in which a specific cytokine signature may contribute to disease severity, as described for DENV.43
5 CONCLUSION
The experience in Venezuela supports a potential role for arboviruses, particularly DENV, as triggers for KD. Further research is required to determine whether the overlapping epidemiological trends and occurrence patterns for arboviruses and KD noted for Venezuela are coincidental, or causal. Understanding the possible interaction and dynamics between arboviruses and the dysregulation of the endothelial bed may lead to an improvement in diagnosis as well as to establish potential prognostic indicators that lead to development of KD.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
AEPM and EMS conceived the study. AEPM, vdAT, MCMC, LADN, OV, and EMS designed the analysis. MMC, LADN, vdAT, and OV designed data collection tools, collected data, and monitored data collection. AEPM, vdAT, MCMC, LADN, OV, and EMS analyzed the data. AEPM, EMS, and vdAT wrote the paper. AEPM, vdAT, MCMC, LADN, OV, and EMS drafted and revised the final version of the paper.




