The safety of antiviral therapy and drug withdrawal for the prevention of mother-to-child transmission of HBV during pregnancy
Li-Xin Xiao and Yi-Ru Chen contributed equally to this work.
Abstract
The efficacy of prenatal antiviral therapy (AVT) for preventing the vertical transmission of hepatitis B virus (HBV) is well demonstrated. However, data are limited regarding the safety of postpartum cessation of AVT, which may induce alanine aminotransferase (ALT) elevation. We aimed to investigate the necessity of prolonging maternal AVT after delivery. Chronic hepatitis B mothers at the immune-tolerant phase with HBV DNA levels >6 log10 IU/mL were prospectively enrolled and received AVT during the third trimester until delivery. Patients were offered to discontinue AVT either at delivery or postpartum week (PPW) 6. In addition, mothers who deferred AVT during pregnancy served as the control group. All mothers were followed until PPW 52 for clinical and virological parameters of hepatitis flares. Among 118 mothers recruited, 91 received AVT with 53 (group A) and 24 (group B) discontinue their treatment at delivery and PPW 6, respectively. Twenty-seven mothers who deferred AVT during pregnancy were followed as the control (group C). A total of 104 of 118 mothers who completed the study, 50% (52/104) had postpartum-elevated ALT levels, which were mild and moderate except 6 of 104 (5.77%) of patients had levels ≥5 times the upper limit of normal; 70% (36/52) of the ALT flares occurred within 12 weeks after delivery. In subgroup analyses, the frequency of ALT elevation was similar among the groups A vs B vs C (50.9% [27/53] vs 58.3% [14/24] vs 40.7% [11/27], respectively; P = .447), as well as the mean peak ALT level (108.4/74.1/126.7 U/L in groups A/B/C, respectively; P = .291). Although postpartum ALT flares were common for mothers with or without AVT during pregnancy, most cases of ALT elevation were mild to moderate. Our study observed that extending AVT to PPW 6 did not affect maternal outcomes and ATV should be discontinued at birth. Close monitoring is warranted as severe flares rarely occurred.
1 INTRODUCTION
Hepatitis B virus (HBV) infection remains a serious clinical problem affecting approximately two billion people worldwide. In the Asia-Pacific region, mother-to-child transmission (MTCT) is the predominant mode of HBV transmission and is associated with an increased risk of developing chronic hepatitis B (CHB).1-5 The implementation of HBV vaccination combined with hepatitis B immune globulin (HBIg) administration has led to substantially decrease the rate of MTCT from 90% to 10%6-8 among mothers with both HBsAg and HBeAg positive. However, despite the combination of active and passive immunoprophylaxis, HBV transmission occurs in 8% to 15% of infants who are born to highly viraemic mothers, which contributes to persistent HBV infection in later life with morbidity and mortality of CHB including cirrhosis and liver cancer.9-12 Maternal treatment with nucleoside analog therapy during late pregnancy is recommended by current practice guidelines to prevent the vertical transmission of HBV in mothers with high levels of viremia.
Currently published studies have thoroughly demonstrated that prenatal administration of nucleoside analogs including lamivudine (LAM), telbivudine (LdT), and tenofovir disoproxil fumarate (TDF) can effectively prevent HBV MTCT among highly viraemic mothers.13-18 It is recommended that oral antiviral therapy (AVT) should be initiated either in the second or third trimester of pregnancy to reduce the viremia before delivery.19-22 However, it is not clear the optimal timing of AVT cessation after delivery while the AVT is no longer needed for the prevention of MTCT. Fewer studies provided data on the safety of AVT withdrawal during postpartum in patients who are at the immune tolerance phase and do not require AVT.
During pregnancy, the placenta, acting as a zone for nutritional exchange and a barrier between maternal and fetal tissues, can catalyze unique maternal immunological and physiological changes to allow tolerance to fetal antigens.24-26 Furthermore, liver metabolism is significantly increased during pregnancy, and the reactivation of HBV owing to immunomodulation overburdens the liver and can even lead to liver failure.27, 28 After delivery, these pregnancy-associated adaptations disappear rapidly, and the immune system is restored. Whether the postpartum immunological alterations and HBV infection rebound after the cessation of AVT has a double implication for alanine aminotransferase (ALT) flares. The proportions of individuals and the time experiencing postnatal flares are discordant in current published studies.13, 29-32 However, fewer data exist on postpartum ALT flares and the optimal duration of AVT is unknown.
We, therefore, performed a prospective study to investigate whether the extension of AVT to postpartum week (PPW) 6 could reduce the frequency and severity of ALT flares when compared to the cessation of antiviral treatment right after birth.
2 PATIENTS AND METHODS
2.1 Study design and patients selection
Pregnant women infected with HBV in immune-tolerant phase and with high viremia during their visit to the Department of Infectious Diseases of the Third Affiliated Hospital of Sun Yat-Sen University, Guangzhou, China, before July 2018, were screened for eligibility to enroll into the study according to the inclusion criteria: age between 18 and 45 years, detectable HBsAg at the screening visit and at least 6 months prior, positivity for serum HBeAg, an HBV DNA level above 106 IU/mL, and an ALT level below the upper limit of normal (ULN; 40 U/L). The exclusion criteria were the following: coinfection with hepatitis A, C, D, or E virus or human immunodeficiency virus; previous AVT for HBV infection (except for antivirals administered to prevent MTCT during a previous pregnancy and discontinued more than 6 months before the current pregnancy); concurrent treatment with cytotoxic drugs, immune modulators, glucocorticoids or nephrotoxic drugs; clinical signs of threatened miscarriage in early pregnancy; evidence of hepatocellular carcinoma or cirrhosis; evidence of fetal deformity by three-dimensional ultrasound examination; the history of congenital malformation or congenital genetic disease in a previous pregnancy; or HBV infection of the husband. The study was approved by the institutional ethics review committee of the Third Affiliated Hospital of Sun Yat-Sen University (Protocol number: [2018]02-269-01) in accordance with the Helsinki Declaration of 1975, and all eligible participants were required to sign an informed consent. This study was registered ClinicalTrials.gov (number: NCT03468907).
2.2 Patient follow-ups and data collections
After discussing the risks and benefits of stopping the therapy right after delivery vs the cessation on treatment at PPW 6, patients were assigned to the aforementioned two groups (A vs B) for observation based on the patient's informed decision. Eligible women were offered the initiation of antiviral treatment with the standard of care options of taking either “LdT” or “TDF” at week 24 of gestation until the postpartum cessation time point specified by the protocol (at delivery in group A vs PPW 6 in group B). During the treatment period, these patients received daily treatment with oral LdT (600 mg Sebivo; Novartis) or TDF (300 mg Viread; Gilead Science). We also enrolled eligible patients who deferred the antiviral treatment as the control (group C) and followed them with the same observation schedule for data comparison. Breastfeeding was recommended to all mothers who had cessation of antiviral treatment after delivery. However, due to the lack of safety data on LdT treatment for mothers with lactation, we did not recommend mothers who opted for group B and taking the AVT until PPW 6.
Before delivery, mothers who opted for AVT were followed up at 4-week intervals after treatment initiation to assess adverse event occurrence and laboratory results (HBV DNA levels, HBV serological status, liver function, hematology, and renal biochemistry) and were followed up again before delivery. In the control group, these assessments were performed at screening and at delivery. After delivery, all subjects, with or without therapy, had the same follow-up schedule: 1, 2, 3, 6, and 12 months postpartum. On the puerperal visits, laboratory data as noted above were collected. Clinical evaluations were performed at baseline, at birth, and also at 1, 3, and 12 months during postpartum follow-ups.
All infants received a 200 IU dose of intramuscular HBIg (Si Chuan Shuyang Company Ltd, Chengdu, China) and the first 10 µg dose of HBV vaccine (Changchun Biotech Research Institute, Changchun, China) within 6 hours after birth. The second and third doses of HBV vaccine were administered at weeks 4 and 24.
2.3 Assessment of outcomes
Appraisals of the safety of the different durations of postpartum AVT were analyzed relative to the AVT regimen according to the following groups: group A = drug withdrawal at delivery, group B = drug withdrawal at PPW 6, and group C = control group. The primary safety outcomes were the assessment of maternal postpartum ALT flares, including the frequency, severity, and initial time of postpartum ALT elevation. The secondary safety assessments included: (a) subgroup analyses of ALT flares and comparison of the aforementioned ALT variables between patients who received TDF vs LdT. (b) Assessment of other adverse events, complications, and abnormal laboratory results among groups. Additionally, the generation of safety reports for infants was assessed during follow-up visits.
In the trial protocol, an increase in serum ALT to the levels equal or greater than five times of the ULN (40 U/L) within 1 year postpartum, was defined as a severe hepatic ALT flare, which required more frequent monitoring and might require the recommencement of AVT if indicated.
2.4 Laboratory measurement and statistic methods
Serum levels of HBV DNA were determined using a COBAS AmpliPrep/COBAS TaqMan HBV test, version 2.0 (Roche Diagnostics), with a detection range of 2 × 108 to 1.7 × 108 IU/mL. HBV serology was determined using an enzyme-linked immunosorbent assay (Shanghai Kehua Bioengineering Co, Ltd, Shanghai, China). Hepatic and renal function was evaluated with an automated bioanalyser (Beckman 8200A). Statistical analysis was performed using the SPSS software (version 20.0; Chicago, IL). Continuous variables were analyzed using one-way analysis of variance and nonparametric tests; categorical variables were analyzed by χ2 and Fisher's exact tests. The significance level was set at P < .05.
3 RESULTS
A total of 118 eligible mothers were enrolled in the trial; the antiviral-treated group contained 87 mothers, including 68 and 19 treated with LdT and TDF, respectively. The remaining 31 subjects who deferred AVT were followed in the control group. However, 14 subjects were excluded from the final data set for analyses, which included one mother withdrew consent before the baseline visit, 9 and 4 mothers were lost to follow-up before delivery and during the postpartum period, respectively. At the time point of 12 months after delivery, 104 mother-infant dyads completing the current study. Participant disposition is shown in Figure 1. Among 77 mothers who received antivirals and were included in the final analysis, 53 had cessation of AVT at delivery (group A), and the 24 who discontinued AVT at PPW 6 were as assigned to group B. In the control group, 27 mothers were included in the current analyses (group C).
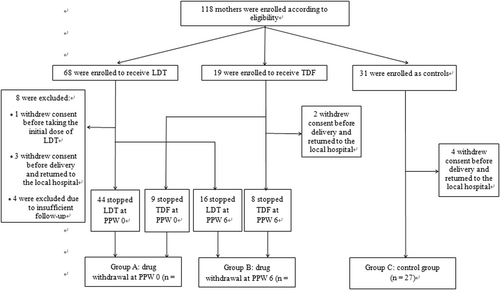
3.1 Demographic and clinical characteristics of the mothers
The baseline demographics and clinical characteristics of each group were similar when compared for gravidity, parity, gestational age, rate of cesarean section, ALT levels before treatment, and rate of the previous AVT at gestational week 24 (Table 1). However, mothers’ mean age (SD) was older in group B, when compared to group A and C (33.30 ± 4.69 vs 30.23 ± 3.30 and 30.81 ± 3.16 years, respectively; P = .004). Mean baseline HBV DNA levels were significantly lower in the control group vs the treatment groups (6.92 ± 0.49 vs 7.31 ± 0.65 log10 IU/mL respectively; P = .007). At delivery, mothers in treatment groups A and B had >3 log10 IU/mL reduction in HBV DNA levels when compared to their baseline levels, while the viral load in the control group did not change significantly from baseline (6.92 ± 0.49 vs 6.83 ± 1.09 log10 IU/mL; P > .05). The mean (±SD) duration of AVT before delivery was comparable with 12.78 ± 2.85 weeks for group A vs 12.81 ± 3.89 weeks for group B (P = .972). All mothers in our study had a singleton pregnancy. In group B, there was a 39-year-old woman who received LdT treatment had a stillbirth. The patient had intrahepatic cholestasis during pregnancy and the stillborn fetus was vaginally delivered at 35 weeks of gestation. The other pregnancy obstetric complications are summarized in Table 1. There were no statistically significant differences in the frequency and severity of gestational diabetes mellitus, intrahepatic cholestasis of pregnancy, premature rupture of membrane, postpartum hemorrhage, polyhydramnios, and oligohydramnios when compared the three groups. One LdT-treated mother in group A developed kidney neoplasms after delivery and was considered not to be AVT-related.
| Characteristics | Group A drug withdrawal at PPW 0 | Group B drug withdrawal at PPW 6 | Group C control group | F or χ2 | P |
|---|---|---|---|---|---|
| Mothers, n | 53 | 24 | 27 | ||
| Age, y | 30.23 ± 3.30 | 33.30 ± 4.69 | 30.81 ± 3.16 | 5.888 | .004 |
| Gravidity, median (rangea) | 1 (1-2) | 1 (1-2) | 1 (1-2) | 0.283 | .868 |
| Parity, median (rangea) | 0 (0-1) | 0 (0-1) | 0 (0-0) | 0.459 | .795 |
| Gestational age, wk | 39.14 ± 1.43 | 39.14 ± 1.56 | 39.33 ± 1.24 | 0.187 | .830 |
| Delivery by cesarean section, n (%) | 20 (37.7%) | 6 (25%) | 11 (40.7%) | 1.593 | .451 |
| Baseline ALT, U/Lb | 22.25 ± 6.71 | 21.83 ± 5.11 | 24.56 ± 7.32 | 1.418 | .247 |
| HBV DNA, log10 IU/mL | |||||
| Baseline | 7.36 ± 0.62* | 7.21 ± 0.72** | 6.92 ± 0.49*** | 4.258 | .017 |
| Before delivery | 3.65 ± 0.73* | 3.00 ± 0.73** | 6.83 ± 1.09*** | 127.885 | .000 |
| Gestational age at AVT, wk | 26.27 ± 2.80 | 26.30 ± 3.65 | NA | ||
| Type of AVT | |||||
| Telbivudine, n (%) | 44 (83.0%) | 16 (66.7%) | NA | ||
| Tenofovir, n (%) | 9 (17.0%) | 8 (33.3%) | NA | ||
| Previous AVTc, n (%) | 2 (3.8%) | 2 (8.3%) | 0 | 2.104 | .258 |
| Obstetric abnormalities | |||||
| Gestational diabetes mellitus, n (%) | 3 (5.7%) | 0 | 1 (3.7%) | 1.087 | .806 |
| Intrahepatic cholestasis of pregnancy, n (%) | 0 | 1 (4.2%) | 0 | 2.765 | .231 |
| Stillbirth, n (%) | 0 | 1 (4.2%) | 0 | 2.765 | .231 |
| Premature rupture of membrane, n (%) | 6 (11.3%) | 2 (8.3%) | 3 (11.1%) | 0.167 | .920 |
| Polyhydramnios, n (%) | 2 (3.8%) | 2 (8.3%) | 0 | 2.104 | .258 |
| Oligohydramnios, n (%) | 6 (11.3%) | 0 | 2 (7.4%) | 2.986 | .225 |
| Postpartum hemorrhage, n (%) | 0 | 2 (8.3%) | 0 | 4.397 | .052 |
| Other adverse events (n) | Kidney neoplasms (1) |
- Note: The plus-minus values are the means ± standard deviations.
- Bold values indicate significant P values.
- Abbreviations: ALT, alanine aminotransferase; AVT, antiviral therapy; HBV, hepatitis B virus; n, number; NA, not available; PPW, postpartum week.
- a Data are expressed as the 25% to 75% interquartile ranges.
- b The upper limit of the normal range for the ALT level in mothers is 40 U/L.
- c All exposures were in the setting of a previous pregnancy for the prevention of mother-to-child transmission, and treatment had been discontinued for more than 6 months before the current pregnancy.
- * t = 25.278; P = .000.
- ** t = 17.992; P = .000.
- *** t = 0.361; P = .720.
3.2 Assessment of maternal postpartum ALT flare
- (a)
Comparison of the frequency of ALT elevation. During the postpartum period, 52 (50%) of the 104 mothers had elevated ALT levels (ie, >40 U/L). However, the difference in the proportion of mothers with postpartum ALT elevation was nonsignificant: 27 of 53 (50.9%) in group A, 14 of 24 (58.3%) in group B, and 11 of 27 (40.7%) in group C, with P = .447 (Table 2). Mild to moderate elevations in the ALT level, that is, from 1.1 to 5 times the ULN, accounted for a significant proportion of the cases of elevated ALT level (46/52, 88.5%).
- (b)
Comparison of the severity of ALT elevation. Six mothers—four in group A and two in group C—experienced a severe hepatic flare (ie, ALT ≥ 5 × ULN). No patients who withdrew therapy at PPW 6 experienced a severe ALT flare, but the difference among the groups was not significant (P = .385). Additionally, the mean maximal ALT level in postnatal 1 year was lower in group B (74.1 U/L) vs group A and control (108.4 and 126.7 U/L, respectively), but the difference was not statistically significant (P = .291).
- (c)
Comparison of the initial time of ALT elevation. Of the 52 patients with elevated ALT levels, 30.8% (16/52) exhibited initial ALT elevation within 1 month after delivery (7/27 [25.9%], 7/14 [50%], and 2/11 [18.2%] in groups A, B, and C, respectively; P = .170). Notably, the overwhelming majority (69.2% [36/52]) had fluctuating ALT levels in the first 3 months postpartum (21/27 [77.8%], 10/14 [71.4%], and 5/11 [45.5%] in groups A, B, and C, respectively; P = .144). The median initial time of ALT elevation was 3 months (interquartile range [IQR]: 1-3 months) for group A, 1.5 months (IQR: 1-6 months) for group B, and 6 months (IQR: 2-12 months) for group C, with F = 2.481; P = .289.
- (d)
Comparison of the clinical outcomes of ALT elevation. Though postnatal ALT elevation was fairly common in this study (52/104 [50%]), the majority of elevated ALT levels naturally subsided (44/52 [84.6%]). The frequency of spontaneous resolution of the flare was comparable across the three groups: 81.5% (22/27) in group A, 92.9% (13/14) in group B, and 81.8% (9/11) in group C (χ2 = 1.000; P = .606). Furthermore, postpartum regression with the pharmaceutical antiviral intervention was observed in the other eight mothers. Serological negativity or seroconversion for HBeAg or HBsAg was not observed in the entire cohort.
| Group A drug withdrawal at PPW 0 | Group B drug withdrawal at PPW 6 | Group C control group | F or χ2 | P | |
|---|---|---|---|---|---|
| Mothers, n | 53 | 24 | 27 | ||
| ALT at baseline, U/La | 22.25 ± 6.71 | 21.83 ± 5.11 | 24.56 ± 7.32 | 1.418 | .247 |
| ALT at delivery, U/L | 22.53 ± 16.24 | 21.79 ± 7.71 | 20.52 ± 7.65 | 0.212 | .809 |
| Postpartum period | |||||
| Elevated ALT levelsb, n (%) | 27/53 (50.9%) | 14/24 (58.3%) | 11/27 (40.7%) | 1.611 | .447 |
| ALT level 1.1 to 5 × ULN, n (%) | 23/53 (43.4%) | 14/24 (58.3%) | 9/27 (33.3%) | 3.250 | .197 |
| Severe ALT flarec, n (%) | 4/53 (7.5%) | 0 | 2/27 (7.4%) | 1.911 | .385 |
| Maximal ALT level in postpartum year 1d | 108.4 ± 96.0 | 74.1 ± 28.1 | 126.7 ± 105.7 | 1.267 | .291 |
| Initial time of elevated ALT level, months postpartum, median (rangee) | 3 (1-3) | 1.5 (1-6) | 6 (2-12) | 2.481 | .289 |
| Elevated ALT level at 1 mo postpartum, n (%) | 7/27 (25.9%) | 7/14 (50%) | 2/11 (18.2%) | 3.546 | .170 |
| Elevated ALT level at 3 mo postpartum, n (%) | 21/27 (77.8%) | 10/14 (71.4%) | 5/11 (45.5%) | 3.877 | .144 |
| Outcomes of postpartum ALT elevation | |||||
| Spontaneous resolutionf, n (%) | 22/27 (81.5%) | 13/14 (92.9%) | 9/11 (81.8%) | 1.000 | .606 |
| Resolution with AVT, n (%) | 5/27 (18.5%) | 1/14 (7.1%) | 2/11 (18.2%) | ||
- Abbreviations: ALT, alanine aminotransferase; AVT, antiviral therapy; n, number; PPW, postpartum week; ULN, upper limit of normal.
- a The upper limit of the normal range for the ALT level in mothers is 40 U/L.
- b An elevated ALT level was defined as an ALT level of greater than 40 U/L.
- c A severe ALT flare was defined as an ALT level of greater than five times the ULN.
- d The maximal ALT level was calculated for mothers who exhibited postpartum ALT elevation during the follow-up period.
- e Data are expressed as the 25% to 75% interquartile ranges.
- f Spontaneous resolution was defined as a decrease in the ALT level to normal without the initiation of AVT.
The demographic and clinical characteristics of these eight mothers, who regressed after AVT, had been summarized. The maximal values of ALT, ranging from 113 to 439 U/L, occurred within 6 months after delivery in 75% (6/8) of these mothers. As mentioned above, six mothers, four in group A and two in group C experienced severe postnatal hepatic flares (ALT ≥ 5 × ULN) and hence recommenced AVT. It was notable that two mothers with a postpartum ALT level of less than 200 U/L—one in group B and the other in group A—were also treated with antiviral medications due to refractory sustainability and the absence of remission.
3.3 Infant outcomes
Among the 103 live birth infants (1/104 mother in group B had a stillbirth), no significant differences were found regarding neonatal growth parameters, including weight, length, and Apgar score at 1 minute. In terms of laboratory variables, no appreciable differences were found in the levels of ALT (12.64 vs 12.86 vs 11.55 U/L, respectively; P = .910), total bilirubin (123 vs 138 vs 158 µmol/L, respectively; P = .465), and albumin (37.15 vs 36.07 vs 38 g/L, respectively; P = .339) across the three groups (Table 3). The frequency of obstetric complications was similar among the three groups, which included the frequency of prematurity, small for gestational age, macrosomia, pneumonia, meconium aspiration syndrome, neonatal hemolytic presentations, asphyxia, hyaline membrane disease, hypoxic-ischemic encephalopathy. In addition, the congenital malformation rate did not differ among the three groups as there were only infants with bilateral pyelonephrosis reported in group B (χ2 = 2.845; P = .223).
| Characteristics at birth | Group 1 drug withdrawal at PPW 0 | Group 2 drug withdrawal at PPW 6 | Group 3 control group | F or χ2 | P |
|---|---|---|---|---|---|
| Infants, n | 53 | 23 | 27 | ||
| Sex, male, n (%) | 28 (52.8%) | 6 (26.1%) | 15 (55.6%) | 5.534 | .063 |
| Weight, kg | 3.12 ± 0.46 | 3.11 ± 0.38 | 3.18 ± 0.50 | 0.165 | .848 |
| Length, cm | 48.91 ± 2.24 | 49.35 ± 1.27 | 49.22 ± 1.81 | 0.433 | .650 |
| Apgar score at 1 min | 9.98 ± 0.15 | 10 | 9.81 ± 0.79 | 1.374 | .259 |
| Laboratory variablesa | |||||
| ALT, U/L | 12.64 ± 9.27 | 12.86 ± 5.87 | 11.55 ± 5.11 | 0.095 | .910 |
| TBil, µmol/L | 123 ± 76.93 | 138 ± 75.35 | 157 ± 29.92 | 0.788 | .465 |
| ALB, g/L | 37.15 ± 2.50 | 36.07 ± 2.98 | 38 ± 2.61 | 1.129 | .339 |
| Neonatal diseases and abnormalities | |||||
| Prematurity, n (%) | 4 (7.5%) | 1 (4.3%) | 0 | 2.222 | .329 |
| Small for gestational age, n (%) | 3 (5.7%) | 0 | 2 (7.4%) | 1.629 | .443 |
| Macrosomia, n (%) | 2 (3.8%) | 1 (4.3%) | 1 (3.7%) | 0.418 | 1.000 |
| Pneumonia, n (%) | 1 (1.9%) | 1 (4.3%) | 1 (3.7%) | 1.052 | .790 |
| Hemolytic disease of the newborn, n (%) | 1 (1.9%) | 0 | 0 | 1.175 | 1.000 |
| Asphyxia, n (%) | 0 | 0 | 1 (3.7%) | 2.524 | .485 |
| Hyaline membrane disease, n (%) | 0 | 0 | 1 (3.7%) | 2.524 | .485 |
| Hypoxic-ischemic encephalopathy, n (%) | 1 (1.9%) | 0 | 0 | 1.175 | 1.000 |
| Congenital malformation (n)b | Bilateral pyelonephrosis (1) | ||||
- Note: Bold values indicate significant P values.
- Abbreviations: ALB, albumin; ALT, alanine aminotransferase; PPW, postpartum week; TBil, total bilirubin.
- a The upper limit of the normal range for the ALT level in infants is 40 U/L.
- b The frequency of congenital malformation among infants in group 2 did not differ significantly from that in group 1 and group 3 (χ2 = 2.845; P = .223).
4 DISCUSSION
Numerous recent clinical trials13-22 have confirmed that AVT during pregnancy can effectively prevent HBV MTCT in highly viremia mothers and increase the success rate of blocking vertical transmission substantially to approach 100%. However, the optimal timing regarding the postpartum cessation of AVT has not been well defined in previous studies. Thus, data are needed to address the health care provider and patients' concerns on hepatitis B flares after the discontinuation of AVT among immune tolerance mothers.23 Our results showed that although postpartum ALT flares are fairly common, most patients only had mild to moderate elevations in the ALT levels within the first 3 months after delivery. More importantly, our study points to the direction of prolonging AVT for 6 weeks after delivery did not change the frequency or severity of ALT flares during the postpartum period. Therefore, it is feasible and safe to stop the antiviral treatment right after delivery on patients who had no further indication for AVT.
Immunological and physiological changes induced after delivery likely influence the liver function of HBV-infected mothers.24-26, 30, 33 Various studies have reported that postpartum ALT abnormality is relatively common: 25% in a study by Giles et al,28 36% in an analysis by ter Borg et al,30 38% in a study by Pan et al.29 Our study observed that 50% (52/104) had exhibited postpartum-elevated ALT levels, which was higher than other published studies. That could be explained by the patient's selections in the current study as we enrolled immune tolerance CHB mothers with an indication of AVT during pregnancy. The majority of mothers in our cohort agreed with AVT and postpartum discontinuation on the treatment. Postcessation of AVT inducing ALT flare have been well documented in many studies.13, 29, 31 However, our observation on the frequency of severe ALT flares (approximate 6%) is consistent with the aforementioned studies on postpartum ALT flares.
The impact of AVT during pregnancy on postpartum ALT flare is not fully understood. Previous studies have reported inconsistent results. The study by Xu et al13 indicated a lower flare rate in the LAM-treated arm than in the untreated arm (25% vs 50%, respectively; P = .002). In addition, of the 52 women with elevated postpartum ALT levels, most (88.5%, 46/52) had mild to moderate elevation, and only six experienced severe flares. Furthermore, the maximal ALT level was 96.7 U/L. Thus, postnatal flares are quite common, and it is sensible and safe to withdraw antivirals after delivery. In our study, the incidence rates of postpartum ALT elevation did not differ appreciably across the three different treatment groups. These findings are consistent with the results reported by Nguyen et al,31 as they observed the similar flare rates among the early cessation group, the late cessation group, and the control group (50% vs 40% vs 29%; P = .33). In contrast, a randomized controlled trial (RCT) by Pan et al29 observed that a significantly higher frequency of ALT elevation in TDF-treated group during pregnancy when compared to mothers without such treatment (60.8% vs 38%; P = .001). Our study had less patients in the control group and may not be able to detect such a difference. Since the RCT by Pan et al29 was not primarily designed for investigating the ALT flares, further large studies are needed to understand the impact of AVT during pregnancy on the postpartum ALT flares. The timing of postpartum AVT cessation remains a controversial topic. Currently, whether postnatal liver flares could be minimized by prolonging the duration of postpartum AVT is unclear, although some studies have investigated the risk factors for postpartum ALT flares. For example, a multicentre observational survey of 241 mother-infant pairs34 in northwestern China, indicated that antiviral withdrawal is prudent for women with elevated ALT levels during pregnancy because these women had a significantly higher rate of ALT flare after LdT withdrawal than those having a normal ALT level throughout pregnancy (25% vs 11.9%, respectively; P = .039). Another study35 presented the argument that early antiviral withdrawal might be related to increased ALT levels. In the current study, we had the opportunity to compare 24 women who received the prolong AVT from delivery to PPW 6 with those who discontinued the AVT at delivery. We found that prolonged treatment did not improve the outcomes and severity of ALT flares when compared to the two groups. This result supported the position of stopping AVT at the time of delivery and the extension of postnatal AVT may be unnecessary. Our results emphasized the necessity of early follow-up and close monitoring of liver function after childbirth, especially during the first 3 months postpartum. Nearly 70% (36/52) of our study participants experienced hepatic flares in the first 3 months postpartum. Other studies that aimed at evaluating the safety of prenatal AVT also found that postnatal flares mainly occur within the first 12 weeks postpartum.31, 33, 34 Recent study by Yi et al32 on mothers without AVT during pregnancy demonstrated that the ALT levels had a bimodal pattern, with peaks at PPWs 3 to 4 and 9 to 12.36 After the withdrawal of prophylactic anti-HBV therapy, women who developed serum ALT flares were observed to be 17.2% within 14 weeks of drug discontinuation in Bzowej's study.37, 38 These flares may arise from the restoration of maternal immunity after delivery, as previously described. Thus, earlier and more careful monitoring is likely important.
In this study, although postpartum ALT elevation was relatively common, many patients (84.6% [44/52]) spontaneously recovered. However, eight women did not exhibit resolution of ALT elevation until antiviral pharmaceutical treatment, six due to severe liver flares and two owing to refractory sustainability and the absence of remission. Regarding the indications and optimal time for the recommencement of AVT, which controls postpartum liver dysfunction after drug withdrawal in HBV-infected pregnant women with high viremia who receive such treatment to prevent MTCT, few reports concerning the relevant studies have been published in the literature to date. Thus, additional evidence-based studies are needed.
Regarding the newborn, according to our investigation, prenatal AVT for preventing the vertical transmission of HBV will not directly affect the growth and development of infants. The incidence of obstetric complications will not be increased. Laboratory tests of the infant's liver and kidney function will not have a direct impact. This suggests that the safety of prenatal use of antiviral drugs for mother-to-child interdiction should be trusted.
This study reflects a detailed real-life, retrospective combined prospective observational survey. However, it has some unavoidable limitations. This study was nonrandomized and did not include patients with CHB in the immune-active phase. In addition, the measurement of a noticeable increase in postpartum liver disease activity is not definitive. In conclusion, during the 12-month postpartum follow-up, postpartum hepatic flares commonly occurred in HBV-infected mothers with high viremia and in the immune-tolerant phase, and most of these ALT elevations were mild to moderate. Thus, it is comparably safe and less aggravating to cease than to continue AVT after delivery. Prolonged administration of AVT after childbirth did not prevent this phenomenon. Additionally, the results of our study indicate that early follow-up and close monitoring are imperative, especially within the first 3 months postpartum. After flares occurred, most individuals spontaneously recovered, and only a very few needed antiviral interventions. The indications and optimal time for AVT recommencement are worthy of further research.
ACKNOWLEDGMENTS
The authors extend sincere appreciation to their patients who agreed to collaborate in this study. This study was funded by Guangdong Province Xin-jiang supporting project (KTP20190272) and the Science and Technology Fund of Guangdong Province (2014A020212483).
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
LX and YC carried out the study and drafted the manuscript. PH and Y-YM collected data and performed data analyses. C-SL and CQP contributed to the study design, data analyses, and critical revision of the manuscript, as well as communication with the journal. All authors had access to the study data and reviewed and approved the final manuscript. LX and YC contributed equally to this study and share the first authorship.




