Sex difference in the development of hepatocellular carcinoma after direct-acting antiviral therapy in patients with HCV infection
Abstract
Sex differences in the predictors for hepatocellular carcinoma (HCC) development after direct-acting antiviral (DAA) therapy was investigated. DAA therapy was given to 1438 (663 male, 775 female) patients. Sex differences in the HCC development rate and the factors contributing to HCC development after DAA therapy were investigated. Male patients had a significantly higher cumulative HCC incidence (log-rank test, P = .007). On multivariate analysis, the fibrosis-4 index (HR = 1.11; 95%CI, 1.042-1.202, P = .002) and posttreatment α-fetoprotein (AFP) (HR = 1.11; 95%CI, 1.046-1.197, P = .001) were found to be independent factors that contributed to HCC development following DAA therapy in female patients, whereas only posttreatment AFP (HR = 1.090; 95%CI, 1.024-1.160, P = .007) was an independent factor in male patients. The optimal posttreatment AFP cut-off values were set based on receiver operating characteristic curve analyses. The optimal posttreatment AFP cut-off value was much higher in females (6.0 ng/mL) than in male (3.5 ng/mL) patients. In conclusion both in male and female patients, posttreatment AFP was an independent predictor of HCC development after DAA therapy. However, the cut-off values differed between the sexes. In male patients, HCC could be seen in patients with relatively low posttreatment AFP levels; more careful observation might be needed in such patients.
Abbreviations
-
- AFP
-
- α-fetoprotein
-
- ALT
-
- alanine aminotransferase
-
- APRI
-
- AST to platelet ratio index
-
- AST
-
- aspartate aminotransferase
-
- AUC
-
- area under the receiver-operating characteristic curve
-
- DAA
-
- direct-acting antiviral
-
- FIB-4
-
- fibrosis-4
-
- HCC
-
- hepatocellular carcinoma
-
- HCV
-
- hepatitis C virus
-
- IFN
-
- interferon
-
- RBV
-
- ribavirin
-
- ROC
-
- receiver-operating characteristic
-
- SOF
-
- sofosbuvir
-
- SVR
-
- sustained virological response
1 INTRODUCTION
Chronic hepatitis C virus (HCV) infection is a major cause of cirrhosis, hepatocellular carcinoma (HCC), and liver failure.1-3 HCC is the third most frequent cause of cancer-related deaths worldwide.1 Eradication of HCV to prevent HCC is the goal of HCV treatment, since viral eradication following interferon (IFN)-based therapy has been found to be associated with a lower risk of HCC in patients with chronic HCV infection.4-6 A sustained virological response (SVR) of greater than 90% has been achieved with very effective direct-acting antiviral agent (DAA) combinations. In a retrospective cohort study of 22 500 HCV patients HCV treated with DAA, the risk of HCC was found to be significantly lower in patients who achieved SVR than in those who did not.7 Moreover, a DAA regimen was found to not be associated with an increased risk of HCC compared to an IFN-based regimen,8 and IFN-based and IFN-free DAA therapies were found to have similar risks of HCC after HCV eradication.9, 10 Nevertheless, HCC can develop even after viral eradication with both DAA and IFN-based therapies.
It has been shown that HCC is male-dominant cancer,11 irrespective of the etiology, and HCC rates are two to four times higher in men.12 In humans, the sex steroid hormones androgen and estrogen are involved in many cellular processes, not only sexual development but also including cellular metabolism and differentiation,13 and both androgen and estrogen receptor α for androgen and estrogen, respectively, appear to have some involvement in liver carcinogenesis.14
In this report, prospective data were reviewed, and the occurrence of HCC after DAA therapy in patients without a history of HCC before DAA treatment was evaluated. The aim of this study was to identify the sex differences in the predictors for the development of HCC after DAA treatment.
2 MATERIAL AND METHODS
2.1 Patients
A total of 1438 consecutive patients infected with HCV genotype 1 or genotype 2 treated with a DAA (IFN-free) regimen between 2014 and 2017 at 10 hospitals belonging to the Ehime Kan-en Network (EKEN net; Ehime University Hospital, Ehime Prefectural Central Hospital, Ehime Prefectural Imabari Hospital, Ehime Prefectural Niihama Hospital, Uwajima City Hospital, Saiseikai Imabari Hospital, Matsuyama Red Cross Hospital, Matsuyama Shimin Hospital, Saiseikai Matsuyama Hospital, and National Hospital Organization Ehime Medical Center) were reviewed. Of the 1438 patients, 641 were treated with ledipasvir and sofosbuvir (SOF) for 12 weeks, and 399 were treated with ribavirin (RBV) and SOF for 12 weeks. Two kinds of RBV were given (Rebetol, Merck Sharp & Dohme, Whitehouse Station, NJ; or Copegus, Chugai Pharmaceutical Co. Ltd, Tokyo, Japan) as oral tablets (600-1000 mg total daily dose, depending on body weight). Dose changes, temporary interruptions, or discontinuation of RBV was performed according to each manufacturer's prescribing information. Overall, daclatasvir and asunaprevir were given to 201 patients for 24 weeks, and ombitasvir, paritaprevir, and ritonavir were given to 112 patients for 12 weeks. The remaining 85 patients received grazoprevir and elbasvir for 12 weeks. A prior history of HCC before starting antiviral therapy excluded patients from this study. Patients on warfarin at the start of DAA therapy were excluded from the analysis of prothrombin activity. For the purposes of the present study, diabetes mellitus was defined as HbA1c more than 6.5% or taking anti-diabetes drugs or insulin preparations before starting DAA therapy.
The Ethics Committee of Ehime University Hospital approved the study protocol (approval ID 1411010), which conformed to the ethical guidelines of the Declaration of Helsinki as amended in 2008. Written, informed consent was obtained from each patient.
2.2 Clinical and laboratory assessments
Clinical and laboratory assessments were performed before treatment. HCV RNA levels were measured using the Roche COBAS TaqMan HCV Auto assay system (Roche Molecular Diagnostics, Pleasanton, CA), with a lower limit of quantification of 1.2 log10 IU/mL. The fibrosis 4 (FIB-4) index was calculated as a surrogate marker of liver fibrosis.15 The score was calculated as follows: FIB-4 index = age (years) × (AST) [IU/L]/(platelet count [109/L] × (ALT [IU/L])1/2). The APRI (AST to platelet ratio index) was also calculated as another marker of liver fibrosis.16 This score was calculated as follows: APRI = AST(/ULN) × 100/platelet count [109/L]. Clinical and laboratory values were also assessed at the end of DAA treatment and analyzed as posttreatment factors.
2.3 Follow-up and diagnosis of HCC
Follow-up of all DAA-treated patients was performed at 3 to 6-month intervals, with monitoring of biochemical and virological markers and blood counts. Ultrasonography (US) or helical dynamic computed tomography (CT) was performed every 3 to 6 months for HCC screening. If new lesions were detected or suspected on US or dynamic CT, magnetic resonance imaging (MRI) or hepatic angiography was performed. The diagnosis of HCC was made based on the presence of typical hypervascular characteristics on angiography, in addition to the dynamic CT or MRI findings. If no typical findings of HCC were seen, fine-needle aspiration biopsy followed by histological examination was performed to confirm the diagnosis.
2.4 Statistical analysis
Differences were evaluated using the χ2-test, Student's t test, or Welch's t-test, as appropriate. Factors that were not normally distributed were evaluated by Welch's t test.
Predictors for HCC development after DAA treatment were evaluated using Cox proportional hazard model analysis or logistic regression analysis. Significant predictive factors that contributed to the development of HCC on univariate analysis were included in the multivariate analysis. Hazard ratios (HRs), odds ratios (ORs), and 95% confidence intervals were also calculated. All P < .05 on two-tailed testing were considered significant. The HCC development rate was calculated using the Kaplan-Meier method. Differences in the rates of new HCC were tested by the log-rank test. Data were analyzed statistically using SPSS software ver. 23 (IBM, Armonk, NY).
3 RESULTS
3.1 Patients’ characteristics
Table 1 shows the patients’ baseline characteristics; there were 663 male and 775 female patients, with an average age at the start of therapy of 65.8 years. Of the patients, 1401 achieved SVR with DAA therapy, and 37 did not.
| Sex (male/female) | 663/775 |
| Age (y) | 65.8 ± 9.9 |
| AST (IU/L) | 49.7 ± 32.7 |
| ALT (IU/L) | 52.1 ± 44.3 |
| Total bilirubin (mg/dL) | 0.8 ± 0.5 |
| Albumin (g/dL) | 4.1 ± 0.4 |
| Prothrombin time (%) | 93.1 ± 16.7 |
| Hemoglobin (g/dL) | 13.7 ± 1.5 |
| White blood cell count (/µL) | 4977 ± 1629 |
| Platelet count (×104/µL) | 15.9 ± 7.3 |
| Body mass index (kg/m2) | 23.3 ± 4.0 |
| AFP (ng/mL) | 10.5 ± 22.4 |
| Total cholesterol (mg/dL) | 170.1 ± 32.4 |
| HbA1c (%) | 5.8 ± 0.9 |
| Diabetes mellitus (no/yes) | 1199/236 |
| HCV RNA (log IU/mL) | 5.9 ± 0.7 |
| IL28B genotype (SNP8099917) (TT/non-TT) | 118/82 |
| SVR/non-SVR | 1401/37 |
| DAA therapy (SOF+LDV/SOF+RBV/ASV+DCV/OBT+PTV+r/EBR+GZR) | 641/399/201/112/85 |
- Note: Data are expressed as means±standard deviation.
- Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; AFP, α-fetoprotein; ASV, asunaprevir; DAA, direct-acting antiviral; DCV, daclatasvir; EBR, elbasvir; GZR, grazoprevir; HCV, hepatitis C virus; LDV, ledipasvir; OBV, ombitasvir; PTV, paritaprevir; r, ritonavir; RBV, ribavirin; RNA, ribonucleic acid; SOF, sofosbuvir; SNP, single-nucleotide polymorphism; SVR, sustained virological response.
3.2 Rates of patients with advanced fibrosis
Advanced fibrosis was defined as the FIB-4 index more than equal to 3.2515, 17 and the APRI more than equal to 1.0.16 The rate of cases with advanced fibrosis was compared between women and men. The rates of advanced fibrosis using the FIB-4 index were 43.1% (335/775) and 40.8% (271/663) in female and male patients, respectively. Similarly, the rates of these patients using the APRI were 35.9% (278/775) and 38.4% (255/663) in female and male patients, respectively. These results showed that the rates of patients with advanced fibrosis were not significantly different using both the FIB-4 index and the APRI (P = .36 and .32 respectively, χ2-test).
3.3 HCC development rate
The median observation period was 803 days after DAA therapy ended, during which 55 cases of HCC developed. The overall incidence of HCC 1, 2, 3, and 4 years after the end of DAA therapy was 2.3%, 3.9%, 4.9%, and 14.4%, respectively (Figure 1A). Figure 1 shows the number of patients who were followed-up to each time point. The incidence of HCC 1, 2, 3, and 4 years after the end of DAA therapy was 1.3%, 2.8%, 3.4%, and 8.8%, respectively, in female patients and 3.2%, 5.2%, 6.7%, and 19.2%, respectively, in male patients, with a significantly higher cumulative incidence of HCC in male patients (log-rank test P = .007; Figure 1B).
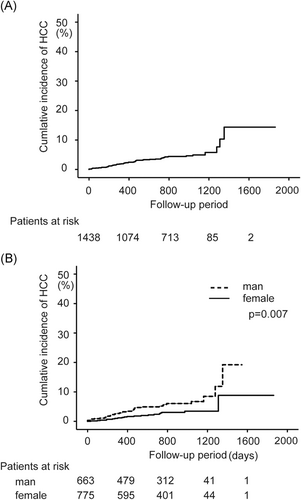
3.4 Predictors for HCC development after the end of DAA therapy in female patients
Pretreatment and posttreatment factors that might contribute to HCC development after the end of DAA treatment were evaluated in female patients. Potential predictors associated with HCC development included sex, age, body mass index, white blood cell count, platelet count, ALT, AST, total bilirubin, albumin, prothrombin time, α-fetoprotein (AFP), total cholesterol, diabetes mellitus, habitual alcohol drinking, the FIB-4 index, the APRI at the start of treatment, and whether SVR was achieved. Posttreatment factors such as the white blood cell count, platelet count, ALT, AST, total bilirubin, albumin, prothrombin time, AFP, and HbA1c were also evaluated.
Univariate analysis identified age (P < .001), white blood cell count (P = .014), platelet count (P < .001), albumin (P < .001), prothrombin time (P < .001), total cholesterol (P < .001), FIB-4 index (P < .001), posttreatment white blood cell count (P = .025), posttreatment AST (P = .034), posttreatment total bilirubin (P = .010), posttreatment albumin (P = .011), posttreatment prothrombin time (P = .001), and posttreatment AFP (P = .027) as predictors (Table 2A).
| HCC | No HCC | P-value | |
|---|---|---|---|
| Age (y) | 72.4 ± 5.7 | 66.7 ± 9.8 | <.001 |
| Body mass index (kg/m2) | 23.4 ± 3.1 | 23.0 ± 4.5 | .71 |
| White blood cell count (/µL) | 3881 ± 1381 | 4753 ± 1550 | .014 |
| Platelet count (×104/µL) | 9.8 ± 4.3 | 16.2 ± 7.6 | <.001 |
| ALT (IU/L) | 46.4 ± 24.9 | 47.9 ± 45.0 | .87 |
| AST (IU/L) | 54.6 ± 21.9 | 49.0 ± 34.4 | .46 |
| Total bilirubin (mg/dL) | 0.9 ± 0.3 | 0.7 ± 0.5 | .17 |
| Albumin (g/dL) | 3.7 ± 0.3 | 4.1 ± 0.4 | <.001 |
| Prothrombin time (%) | 80.8 ± 15.5 | 95.1 ± 17.0 | <.001 |
| AFP (ng/mL) | 23.4 ± 27.5 | 10.2 ± 21.5 | .066 |
| Total cholesterol (mg/dL) | 143 ± 28.1 | 176 ± 33.5 | <.001 |
| Diabetes mellitus (no/yes) | 16/4 | 670/84 | .27 |
| HCV RNA (log IU/mL) | 5.9 ± 0.6 | 6.0 ± 0.7 | .078 |
| Alcohol (none/drinking/unknown) | 15/0/5 | 541/39/195 | .58 |
| FIB-4 index | 7.2 ± 4.0 | 3.8 ± 3.9 | <.001 |
| APRI | 1.9 ± 1.2 | 1.1 ± 2.3 | .13 |
| Posttreatment white blood cell count (/µL) | 4003 ± 1492 | 4830 ± 1588 | .025 |
| Posttreatment ALT (IU/L) | 21.8 ± 8.0 | 20.3 ± 15.2 | .65 |
| Posttreatment AST (IU/L) | 32 ± 9.7 | 25.9 ± 12.2 | .034 |
| Posttreatment total bilirubin (mg/dL) | 1.0 ± 0.4 | 0.7 ± 0.4 | .010 |
| Posttreatment albumin (g/dL) | 3.8 ± 0.3 | 4.1 ± 0.3 | .011 |
| Posttreatment prothrombin time (%) | 78.4 ± 18.9 | 94.8 ± 18.3 | .001 |
| Posttreatment AFP (ng/mL) | 9.1 ± 8.9 | 4.6 ± 3.7 | .027 |
| SVR/non-SVR | 19/1 | 741/14 | .32 |
| (B). Independent factors associated with the development of HCC after DAA treatment on cox proportional hazard model analysis in female patients | |||
|---|---|---|---|
| Hazard ratio | 95%CI | P-value | |
| FIB-4 index | 1.119 | 1.042-1.202 | .002 |
| Posttreatment AFP | 1.119 | 1.046-1.197 | .001 |
- Note: Data are expressed as means ± standard deviation.
- Abbreviations: ALT, alanine aminotransferase; AFP, α-fetoprotein; APRI, AST to platelet ratio index; AST, aspartate aminotransferase; FIB-4, fibrosis-4; SVR, sustained viral response.
Factors included in the multivariate analysis were the FIB4 index, total cholesterol, posttreatment white blood cell count, posttreatment AST, posttreatment total bilirubin, posttreatment albumin, posttreatment prothrombin time, and posttreatment AFP. Platelet count and age and were excluded because they are included in the FIB-4 index and might, therefore, be confounders. On multivariate analysis, posttreatment AFP (HR = 1.119; 95%CI, 1.046-1.197, P = .001) and the FIB-4 index (HR = 1.119; 95%CI, 1.042-1.202, P = .002) were found to be independent factors contributing to HCC development (Table 2B).
3.5 Predictors for HCC development after the end of DAA treatment in male patients
Pretreatment and posttreatment factors that might contribute to HCC development after the end of DAA therapy were evaluated in male patients, and the potential predictors were the same as for female patients.
On univariate analysis, age (P = .046), albumin (P = .019), platelet count (P = .005), white blood cell count (P = 0.017), prothrombin time (P < .001), FIB-4 index (P < .001), APRI (P = .005), posttreatment prothrombin time (P = .013), posttreatment albumin (P = .009), and posttreatment AFP (P = .007) were found to be predictors (Table 3A).
| HCC | No HCC | P-value | |
|---|---|---|---|
| Age (y) | 68.0 ± 7.9 | 64.5 ± 10.1 | .046 |
| Body mass index (kg/m2) | 23.8 ± 3.4 | 23.7 ± 3.5 | .87 |
| White blood cell count (/µL) | 4612 ± 1527 | 5302 ± 1673 | .017 |
| Platelet count (×104/µL) | 12.5 ± 5.9 | 15.9 ± 7.0 | .005 |
| ALT (IU/L) | 56.3 ± 29.7 | 57.0 ± 44.2 | .93 |
| AST (IU/L) | 58.4 ± 30.4 | 50.0 ± 31.0 | .11 |
| Total bilirubin (mg/dL) | 0.8 ± 0.4 | 0.8 ± 0.6 | .81 |
| Albumin (g/dL) | 3.9 ± 0.4 | 4.1 ± 0.3 | .019 |
| Prothrombin time (%) | 84.4 ± 15.2 | 91.6 ± 15.9 | <.001 |
| AFP (ng/mL) | 12.1 ± 10.0 | 10.3 ± 23.8 | .67 |
| Total cholesterol (mg/dL) | 165 ± 31.5 | 164 ± 29.8 | .85 |
| Diabetes mellitus (no/yes) | 25/10 | 488/138 | .40 |
| HCV RNA (log IU/mL) | 5.7 ± 0.7 | 5.9 ± 0.8 | .10 |
| Alcohol (none/drinking/unknown) | 16/11/8 | 320/139/204 | .49 |
| FIB-4 index | 5.4 ± 3.6 | 3.3 ± 2.5 | <.001 |
| APRI | 1.8 ± 1.6 | 1.1 ± 1.2 | .005 |
| Posttreatment white blood cell count (/µL) | 4931 ± 1538 | 5499 ± 1765 | .085 |
| Posttreatment ALT (IU/L) | 24.6 ± 17.3 | 24.9 ± 28.7 | .95 |
| Posttreatment AST (IU/L) | 29.8 ± 17.5 | 27.5 ± 32.6 | .69 |
| Posttreatment total bilirubin (mg/dL) | 0.8 ± 0.3 | 0.8 ± 0.5 | .52 |
| Posttreatment albumin (g/dL) | 3.9 ± 0.3 | 4.1 ± 0.3 | .009 |
| Posttreatment prothrombin time (%) | 82.7 ± 13.4 | 92.1 ± 18.5 | .013 |
| Posttreatment AFP (ng/mL) | 7.3 ± 5.7 | 4.4 ± 3.7 | .007 |
| SVR/non-SVR | 33/2 | 608/20 | .32 |
| (B). Independent factors associated with the development of HCC after DAA treatment on cox proportional hazard model analysis in male patients | |||
|---|---|---|---|
| Hazard ratio | 95%CI | P-value | |
| Posttreatment AFP | 1.090 | 1.024-1.160 | .007 |
- Note: Data are expressed as means ± standard deviation.
- Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; AFP, α-fetoprotein; FIB-4, fibrosis-4; APRI, AST to platelet ratio index; SVR, sustained viral response.
Multivariate analysis identified posttreatment AFP (HR = 1.090; 95%CI, 1.024-1.160, P = .007) as the independent factor that contributed to the development of HCC (Table 3B). Factors included in the multivariate analysis were the FIB-4 index, white blood cell count, posttreatment albumin, posttreatment prothrombin time, and posttreatment AFP. Age and platelet count were excluded from the multivariate analysis, as in the analysis of female patients.
3.6 Cut-off values of posttreatment AFP to predict HCC incidence after DAA treatment in female patients
Optimal cut-off values were set in accordance with the receiver operating characteristic (ROC) curves (Figure 2A). The optimal cut-off value of the posttreatment AFP in female patients was 6.0 ng/mL, with a sensitivity of 54.5% and specificity of 76.3%. On the log-rank test, cumulative HCC incidence was significantly higher in patients whose posttreatment AFP was more than equal to 6.0 ng/mL than in patients whose posttreatment AFP was less than 6.0 ng/mL (P = .023; Figure 3A). However, HCC incidence was not significantly different between patients whose posttreatment AFP was more than equal to 4.0 ng/mL and less than 4.0 ng/mL (P = .37; Figure 3B), or similarly between patients whose posttreatment AFP was more than equal to 5.0 ng/mL and less than 5.0 ng/mL (P = .066).
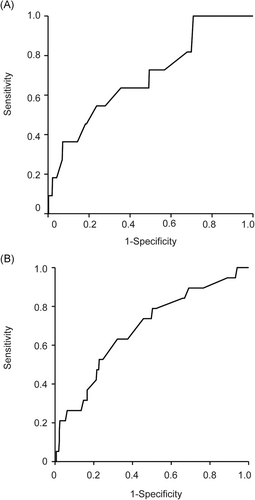
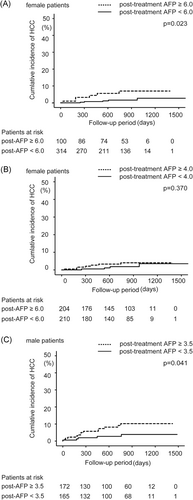
3.7 Cut-off values of posttreatment AFP to predict HCC incidence after DAA treatment in male patients
Optimal cut-off values were set in accordance with the ROC curves (Figure 2B). The optimal cut-off value of the posttreatment AFP in male patients was 3.5 ng/mL, with sensitivity of 73.7% and specificity of 52.2%. On the log-rank test, cumulative HCC incidence was significantly higher in patients whose posttreatment AFP was more than equal 3.5 ng/mL than in patients whose posttreatment AFP was less than 3.5 ng/mL (P = .041; Figure 3C).
3.8 Factors related to posttreatment AFP less than 3.5 ng/mL in male patients
As indicated above, the cut-off values for HCC incidence after DAA treatment were lower in males than in female patients. An additional analysis was performed to identify which population of male patients has a posttreatment AFP of less than 3.5 ng/mL. Thus, factors that might contribute to posttreatment AFP less than 3.5 ng/mL in male patients were evaluated. Potential predictors associated with HCC development included sex, age, body mass index, white blood cell count, platelet count, α-fetoprotein (ALT), AST, total bilirubin, albumin, prothrombin time, total cholesterol, diabetes mellitus, habitual alcohol drinking, the FIB-4 index, and the APRI at the start of treatment.
Univariate analysis identified body mass index (P = .039), platelet count (P < .001), ALT (P < 0.001), AST (P < .001), albumin (P < .001), prothrombin time (P < .001), APRI (P < .001), and FIB-4 index (P < 0.001) as predictors for posttreatment AFP less than 3.5 ng/mL in male patients (Table S1A).
Multivariate analysis identified albumin (OR = 0.25; 95%CI, 0.116-0.536, P < .001) and prothrombin time (OR = 0.97; 95%CI, 0.954-0.991, P = .003) as independent factors related to posttreatment AFP less than 3.5 ng/mL in male patients (Table S1B). The factors included in the multivariate analysis were body mass index, albumin, prothrombin time, and the FIB-4 index.
4 DISCUSSION
In this study, the cumulative HCC incidence after DAA therapy was significantly higher in male than in female patients, and the sex differences in the predictors for the development of HCC after DAA therapy were identified. In female patients, the FIB-4 index and posttreatment AFP were the independent predictors on multivariate analysis, whereas in male patients, only posttreatment AFP was the independent predictor of HCC occurrence after DAA therapy. posttreatment AFP was a predictor in both male and female patients, but the cut-off values of the posttreatment AFP differed between them. In male patients, the cut-off value was 3.5 ng/mL, lower than in female patients. These results probably mean that the effect of decreased AFP on carcinogenesis after HCV elimination by DAA therapy is greater in male than in female patients.
A gene located on chromosome 4 encodes AFP, the major serum protein during fetal life.18 Shortly before birth, albumin replaces AFP as the major serum protein,19 and serum AFP levels then remain very low (<10 ng/mL) throughout life. AFP measurement is widely used as a serological marker for some tumors, since patients with germ-cell tumors, and HCC frequently have increased serum AFP levels.6 However, patients with chronic viral hepatitis and cirrhosis without HCC also often have increased AFP levels.20 Though liver inflammation in patients with viral hepatitis may be one explanation for these elevations of AFP levels, the relationships between levels of AFP and of liver inflammation markers such as alanine aminotransferase (ALT) remain unclear.
In IFN-based therapy, posttreatment ALT and AFP levels were found to be significantly associated with hepatocarcinogenesis, and higher posttreatment ALT and AFP levels after these therapies increased the risk of HCC development in a cohort of 1,818 patients.21 AFP at 24 weeks after the end of IFN-based therapy was also found to be associated with HCC development.22 AFP cut-off values after IFN-based therapies in these reports were 6.0 and 5.0 ng/mL.21, 22 With DAA treatment, we recently reported that multivariate analysis including both pre- and posttreatment factors showed that a higher posttreatment AFP and a higher FIB-4 index were independent risk factors for HCC development, as shown in an earlier study of IFN-based therapy, with a cut-off value for posttreatment HCC of 6.0 ng/mL.23 Similarly, lack of reduction of AFP during DAA therapy was shown to be a predictor of the development of HCC, with 6.0 ng/mL as the cut-off value.24
According to some past reports, the androgen receptor (AR) was found to be expressed in HCC and surrounding non-HCC tissues.25, 26 These findings might indicate that AR activation is associated with hepatocarcinogenesis and cancer development. In fact, it has been reported that the recurrence rate is higher in the AR expression group than in the AR non-expression group, and, moreover, AR expression in HCC tissues is associated with a poor prognosis.27 AR is known to be activated in both ligand-dependent and ligand-independent manners. The latter is through mitogen-activated protein kinase (MAPK), v-akt murine thymoma viral oncogene homolog1 (Akt), and signal transducer and activator of transcription (STAT),28 which are known to be growth and survival pathways. Moreover, it has been reported that more than 200 gene expression levels were changed by AR treatment, and in the liver, these included transforming growth factorβ and vascular endothelial growth factor, which have been implicated as the key pathways in the progression of HCC.29, 30
In the present study, HCC developed in male patients whose posttreatment AFP level was relatively lower than in female patients. In the present patients, before treatment and posttreatment AFP levels were not significantly different between female and male patients. In addition, as described in the results, the rate of advanced liver fibrosis was not significantly different. As shown in Tables 2 and 3, alcohol drinking was not related to HCC occurrence in both female and male patients. Thus, it was thought that these results would be at least partially due to the activation of AR signaling associated with cancer development. However, there were also reports against the association of AR signaling and cancer development.31 Multivariate analysis showed that posttreatment AFP less than 3.5 ng/mL was affected by serum albumin and prothrombin time, which reflect protein synthesis ability and are markers of hepatic functional reserve. However, the actual reason for the lower cut-off level in male patients is not clear.
In conclusion, HCC development after DAA treatment could be seen in male patients even with relatively low posttreatment AFP levels. In the present cohort, the cut-off AFP value was 3.5 ng/mL, lower than in some past reports. In male patients, very careful observation is needed after DAA treatment.
ACKNOWLEDGMENTS
This work was partially supported by JSPS KAKENHI (No. JP18K15818) to Takao Watanabe and JSPS KAKENHI (No. JP18K08007) to Yoichi Hiasa.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTION
Study design: TW, YT, MA, and YH. Data acquisition: TW, KJ, KM, NH, YT, FT, YK, SN, KY, YN, and YH. Quality control of data and algorithms: TW, YT, MH, and YH. Data analysis and interpretation: TW, YT, MH, and YH. Statistical analysis: TW, AY, MH, and YH. Manuscript preparation: TW and YH. Manuscript editing: TW and YH. Manuscript review: MA and YH.




