Local anesthetic improves individuals affected with herpes simplex type 1 labialis
Abstract
Infections caused by the herpes simplex virus 1 (HSV-1), commonly called herpes simplex labialis (HSL), are a public health problem, reaching around 40% of the world's population. Thus, the search for effective therapeutic alternatives in the control of the limitations caused by this virus during the stages of evolution of the disease, is necessary, since they have a direct impact on the quality of life of the patients. The aim of the present study was to evaluate the efficacy of the in situ film precursor semisolid composition in the treatment of herpes simplex lesions in human HSV-1. Ninety-eight (n = 98) patients with HSV-1 were used for this study. The initial exclusion criteria left 81 patients to be considered in the present study. Three applications were performed, the first at time zero (T0) and the other two at 8 and 16 hours, after initial application (T8 and T16). Photographs were taken in the first appointment and 24 and 72 hours after the last application. After the three periods, each patient received a total amount of 90 mg of anesthetic and the prognosis of the patients was followed for 6 months and 1 year after the application. Frequency analysis showed that 40.3% of patients had remission of symptoms 24 hours after the last application. For the present study, the film presented a positive therapeutic potential and an esthetic benefit that is absent in the current products (ointments and gels). The invent presents dosage convenience (only three applications in a 24-hour period) and a low production cost, with a much shorter healing time than that reported using topical antiretrovirals.
Highlights
-
Herpes simplex virus (HSV-1) can be easily treated by an in situ film precursor semisolid
-
The film demonstrated low cost of production and an easy application for the patients
The proposed new treatment promoted a psychosocial improvement and well-being of the participants, against a pathogeny so inconstant and with peculiar characteristics.
-
The present treatment have an effective decrease in the signs and symptoms of the herpetic lesions of the labial and peri buccal lesions.
-
New treatment with a much shorter healing time than that reported using regular topical antiretrovirals.
1 INTRODUCTION
Herpes simplex virus (HSV) infections continue to be a public health problem.1-3 Herpes viruses are highly contagious and are classified as: type 1, inducer of extragenital clinical conditions, especially the upper body, especially the skin, oral mucosa, oropharyngeal and conjunctiva, trigeminal ganglion and nervous system; and type 2, inducer of genital and perigenital clinical conditions and other lower regions of the body, such as the skin and sacral ganglia.4
Virus transmission occurs by direct contact with secretions from infected areas, enters the host through the mucosa, membranes, or injured skin and is then transported along the peripheral sensory neurons to the nodes where they remain in the nonreplicating state.5-11
Recurrent infections can be triggered by various stimuli, such as stress, fever, sun exposure, temperature extremes, ultraviolet radiation, immunosuppression, or trauma.12 HSV-1 generally occurs one to six times a year.13 A small proportion of patients have outbreaks that occur monthly or more frequently.14 These recurrent infections could be related to HSV resistance to antivirals.15
In the case of recurrent cold sores, six stages are defined: prodromal, erythema, papule or edema, ulcer, crusting, and scarring. Theauthors also demonstrated that symptoms of tingling, pain, paresthesia, pruritus, and burning precede injuries of herpes in 60% of patients.13 Then, the lesions appear as papules that evolve into vesicles and blisters filled by inflammatory exudate, the stage in which there is a greater probability of transmission.8, 16-19
Local anesthetics are attributed: (a) anti-inflammatory action superior to nonsteroidal and some steroidal anti-inflammatory agents, and reduced side effects20; (b) antimicrobial action: they are considered adjuvants or alternatives to traditional antimicrobial agents due to the ability to destructure or increase the permeability of the cell membrane21, 22; and c) antiviral control.20
In the double-blind, sequential, placebo-controlled study developed by Cassuto,23 patients with herpes simplex types 1 and 2 were treated with local topical anesthetic cream (lidocaine/prilocaine) and the duration of symptoms in phases prodromal and active disease. In the prodromal period, the duration of symptoms was reduced by about 60% for type 1% and 65% for type 2, and in the active phase, the effect was even more pronounced, with a reduction of 65% and 75% for types 1 and 2, respectively.23
The present study aims to evaluate the efficacy of a semisolid film composition in situ for the treatment of patients with herpetic lip lesions.
2 MATERIALS AND METHODS
The project was approved by the Research Ethics Committee of the School of Dentistry of Ribeirao Preto, University of São Paulo (USP), via Brazil Platform (Brasil Plataforma, Federal government, Brazil), under the CAAE process: 45356915.6.0000.5419—and REBEC platform—http://www.testeclinicos.gov.br/ with registry RBR-4kkq8c that follows the international precepts about human use in research.
Ninety-eight (n = 98) patients with Recurrent Herpes Simplex Lips were invited to participate in the study by means of an announcement at local Radio-USP and posters at the Units of Ribeirao Preto-USP. The purpose of the project and the procedures to be carried out were explained. The preselected participants were then attended by the first author of the present study under the strict guidance of the last author of the present study.
On the selection of patients, criteria based on a study by Khemis et al24 that defines inclusion and exclusion criteria. Inclusion criteria: this study included legal or minor adults with accompanying legal guardians of both genders, immunocompetent, including smokers and alcoholics. Exclusion criteria: participants in use or who used systemic-specific antiviral medication in the 15 days preceding the study, as well as pregnant women and patients with heart disease were not included in the study.
The present study presented five questionnaires that define the inclusion, exclusion, and satisfaction of individuals in the treatment (Figure 1). After the first questionnaire (Q1), 11 individuals were randomly excluded from having systemic disease and six individuals were excluded because they did not send questionnaire 2 (Q2).
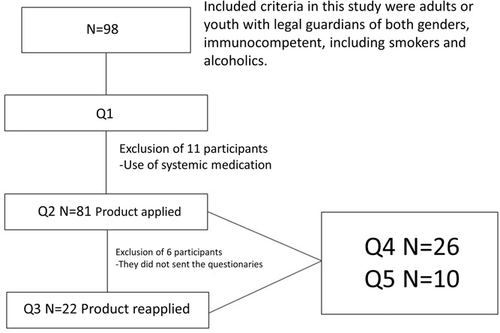
Therefore, for the present study, 81 (n = 81) eligible individuals were passed to the application of the film and were distributed demographically according to Table 1.
| Features | N | % |
|---|---|---|
| Genre | ||
| Male | 21 | 25.9 |
| Female | 60 | 74.1 |
| Skin color | ||
| White | 70 | 86.4 |
| Pardo | 10 | 12.3 |
| Yellow | 1 | 1.2 |
| Schooling | ||
| 12 y or older | 73 | 90.1 |
| Between 8 and 12 y | 5 | 6.2 |
| Between 8 and 4 y | 2 | 2.5 |
| Up to 1 y | 1 | 1.2 |
| Family income | ||
| Up to 1 minimum wage | 2 | 2.5 |
| 1 to 5 minimum wages | 38 | 46.9 |
| 5 to 10 minimum wages | 21 | 25.9 |
| More than 10 salaries | 20 | 24.7 |
2.1 Formulations and methods
A rational drug proposal contemplated two factors for the present study: (a) pharmacological actions attributed to the drug(s), minimizing or avoiding the inflammatory process, acting in the fight against herpes and in cases of herpetic wound, reduce contamination bacterial25; and (b) relate to the formulation, the use of biocompatible, nonirritating, bioadhesive, and film-forming polymers,26 which make it possible to obtain in situ film-forming precursor gel. The formulation contains a mixture of anesthetics prilocaine and lidocaine, and the adjuvants used are all approved for use in topical compositions by regulatory agencies. These compositions were deposited in the National Institute of Industrial Property (INPI BR 10 2018 073081-9).
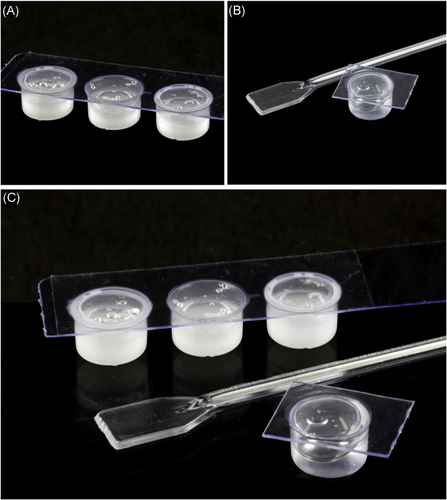
Figure 2 shows the formulations used to make the work feasible. Each kit was labeled with the batch and date of the container and containing: three doses of F1 (with drug)—180 mg (Figure 2A), previously weighed in precision scale; a dose of inert formulation (without the drug)—180 mg (Figure 2B); and four disposable spatulas without tips. In addition, we have the demonstration of Figure 2C, where we can see the comparison of the tonalities of the presented products. The patient was instructed to keep the kit cool at 4°C for the entire study period (16 hours maximum T0 to T16—explained bellow) with the sealed side facing up.
The inert formulation was used to teach the participant how to apply the paste to the lesion. The clinical application of the film precursor semisolid composition with the active principle was performed in three moments. All the 81 patients applied the product at time zero (initial consultation—T0) and the other two applications at 8 and 16 hours after initial application (T8 and T16), in case of relapse (n = 22) the same treatment were instructed (T0-R, T8-R, and T16-R). At each time, an amount of formulation equivalent to 180 mg or 0.18 g containing 30 mg of anesthetic, that is, 15 mg of each anesthetic salt (active principles) was applied and spread in the region where pruritus/burning was occurring (prevesicular). Thus, the total anesthetic applied in 16 hours was 90 mg. After the first application, the participant was instructed to maintain the semisolid composition at the lesion site for at least 1 hour, until the formation of the transparent film, whose time should be recorded. After 60 minutes, the film could be removed.
In all applications, the participant was advised to record (a) ease of application, time for induction of anesthesia and its duration, and whether the sensation of anesthesia bothers the patient and (b) the time of remission of herpes symptoms. In cases where the manifestation is in the postvesicular phase, the gel was applied on the vesicle and adjacent. After 24 hours of the first application, the researcher contacted the participant through links to check the evolution and completion of the Q2 (n = 81): an experiment on application of the new formulation, divided into four parts: F) characterization of the lesion; G) aspects and sensations about the new formulation; H) results of treatment with the new formulation; I) psychosocial influence after application of the new formulation.
If, during this period, a recurrence of Herpes occurred, the participant underwent the treatment and answered the Q3 (n = 22): treatment with new formulation after relapse during the study period.
To conclude, all participants were submitted to satisfaction questionnaires (follow-ups satisfaction of 6 months and 1 year), the first being performed after 6 months, questionnaire 4 (n = 26): satisfaction on the new treatment and the second after 1 year, questionnaire 5 (n = 10): for validation of the previous. The second follow-up satisfaction questionnaire was developed using the Likert scale as a reference for the response alternatives27 (Figure 1).
Continuous variables were expressed in terms of mean and standard deviation. The paired t test was used to compare the times. To determine the correlation between variables, the Pearson correlation coefficient was used. Fisher's exact test and Pearson's χ2 were used to compare the ordinal qualitative variables. The level of statistical significance of 5% was adopted in all cases.
3 RESULTS
From 81 patients whom were submitted to the initial application of the product (T0, T8, and T16), 22 searched the research team for a new application due from recurrences after starting treatment with the new formulation. From the 22 patients, 19 (86.4%) participants had only one recurrence, one (4.5%) three recurrences, and two (9.1%) had four recurrences. Fifteen (68.2%) reported that the site of the new lesion was not the same as the treatment with the new formulation at some other time, and seven (31.8%) reported that the site coincided site of the first application. From our findings, our research group could infer that our product is effective and the present formulation have a rate of the recurrence one-third (34.1%), which is a very low rate of recurrence.
When comparing the patient's satisfaction with the applications, it was verified that there was a significant difference between the applications, with an increase in satisfaction with each application T0 to T16, when it compares the time of the experiments T0 vs T16 with “no”—“(D) discomfort” (Table 2, part 1). An increase of 46.9% on T0 to 71.6% on T16 shows that the anesthetic does not make discomfort at all to more that two-thirds of the participants and no discomfort at all to the participants after T16. Other parameters of Table 2 shows that the “very much” satisfaction with the formulation was increased from 19.8% on T0 to 32.1% on T16. The present table also shows a decrease of “Reasonable” comparing to discomfort (D) from 53.1% on T0 to 1.2% on T16 (Table 2, part 1).
| Percentage of the participants regarding the aspect of the film during the application of the new formulation | ||||||
|---|---|---|---|---|---|---|
| T0 | T8 | T16 | ||||
| Part 1 | S | D | S | D | S | D |
| No | 4.9% (4) | 46.9% (38) | 2.5% (2) | 60.5% (49) | 3.7% (3) | 71.6% (58) |
| Little | 22.2% (18) | 30.9% (25) | 18.5% (15) | 29.6% (24) | 12.3% (10) | 22.2% (18) |
| Reasonable | 53.1% (43) | 17.3% (14) | 51.9% (42) | 8.6% (7) | 51.9% (42) | 1.2% (1) |
| Very much | 19.8% (16) | 4.9% (4) | 27.2% (22) | 1.2% (1) | 32.1% (26) | 0.0% (0) |
| Percentage of the participants regarding the sensation of anesthetic during the application of the new formulation | ||||||
|---|---|---|---|---|---|---|
| Part 2 | T0 | T8 | T16 | |||
| No | 2.5% (2) | 6.2% (5) | 9.9% (8) | |||
| Weak | 25.9% (21) | 34.6% (28) | 43.1% (35) | |||
| Regular | 43.2% (35) | 49.4% (40) | 40.7% (33) | |||
| Strong | 28.4% (23) | 9.9% (8) | 6.2% (5) | |||
- Note: The metrics of the aspect of the film were measured as “no,” “little,” “reasonable,” and “very much.” Part 2 demonstrates the percentage of the participants regarding the sensation of anesthetic during the application with the personal sensation as “no,” “weak,” “regular,” and “very strong” anesthetic sensation during the time of the experiment (T0, T8, and T16). Data in percentage (number of participants) and N = 81.
In Part 2 of Table 2, the table demonstrates a sensitive increase of “No sensation of anesthesia” between T0—2.5% to T16—9,9%, but the present table also shows that the effect of the strong anesthesia decrease abruptly from T0—28.4% to T16—6.2%.
Table 3 presents the evaluation of the participants' satisfaction with the aspect of the film formed, after each application, and the result of the assessment of the anesthetic sensation after each application of the new formulation, on the use of the new formulation (Table 3 [question 1]). Table 3 also presents the participants' assessment of the intensity of anesthetic sensation after each application of the new formulation. Regarding the discomfort of the anesthetic sensation, there was a significant difference between the applications with decreased discomfort at each application (Table 3 [question 2]). In addition, for the sensation caused by anesthesia, there was a significant difference in intensity at each application, with a decrease of the sensation (Table 3 [question 3]).
| T0 vs T8 (**) | T0-R vs T8-R (**) | T8 vs T16 (**) | T8-R vs T16-R (**) | |
|---|---|---|---|---|
| 1) Is the aspect of the film satisfactory? | <.001* | .001* | <.001* | <.001* |
| 2) Does the anesthetic sensation generate discomfort? | <.001* | <.001* | <.001* | <.001* |
| 3) What is the intensity of the sensation caused by anesthesia? | <.001* | <.001* | .006* | <.001* |
| first application vs relapse application | ||||
| 4) Did the treatment with the new formulation cause any irritation or discomfort? | P = .429 | |||
- Note: Better results are shown as (**), when comparing T0 to T8 and to T16 or T0-R to T8 and to T16-R, the findings suggests that the film 1) increases in aspects of satisfaction, 2) generates less discomfort in time, 3) less intensity of anesthesia in time. Comparing irritation and discomfort between the 4) first and second applications, no difference was found, P = .429, which means no discomfort on the first and the second application.
- * Pearson's χ2 test.
There was no change in the feeling of irritation or discomfort with the use of the new formulation in participants who had relapses (Table 3 [question 4]).
When evaluating the aspects and sensations about the new formulation after each application (Table 3), it was verified that there was a significant difference in the satisfaction regarding the aspect of the film between the applications, with increased satisfaction to each application (T0 vs T8 and T8 vs T16/T0-R vs T8-R and T8-R vs T16-R). For the discomfort caused by the anesthetic sensation, there was a significant difference and the discomfort decreased in the cycle of relapse. There was also a significant difference between the applications, with decreased discomfort for each application.
Table 4 shows the improvement of the psychosocial aspects after the use of the new formulation (questionnaire 1—no application of the film vs questionnaire 2—application of the film), which was significant for all the questions, except for the change of routine habits, which was almost different (P = .052). There was a positive correlation between the time the lesion disappeared alone and the number of applications of the formulation necessary to eliminate the symptoms.
| Q1 vs Q2* | |
|---|---|
| Modifies routine habits | .052 |
| Stop leaving home for leisure | <.001 |
| Depression | <.001 |
| Embarrassment | <.001 |
| Harms professional performance | <.001 |
- * Pearson's χ2 test.
4 DISCUSSION
The use of local anesthetics for the purpose of desensitization and prevention of pain sensation represents one of the great advances in the area of human health. The therapeutic benefit of antiviral cream formulations containing acyclovir, penciclovir, docosanol, foscarnet, vidarabine (adenine arabinoside), edoxudine, and idoxuridine remains questionable,28 which leaves the present study with positive results compared to studies with these agents in these presentations. It is also worth noting that compared to topical gels, the use of the new formulation showed a significant reduction in psychosocial aspects in relation to herpes. It is suggested that this better evaluation results from the esthetically favorable aspect of the medication, besides accelerating improvement of the symptoms and reducing the healing time.
Although evidence suggests that oral antiviral agents are more beneficial than topical agents for the treatment of recurrent episodes, their use is not frequent, as most of these drugs are available only by prescription.29 Clinical studies have demonstrated that these products provide a small clinical benefit by reducing the duration of symptoms.29 Studies of Spruance et al30 and Femiano et al31 showed that the topical treatment with penciclovir cream showed improved therapeutic results compared to acyclovir cream.
Regarding this new formulation, most patients started the treatment in the closed vesicle stage (44.4%), 38.3% when the symptoms lasted 1 day and only 17.3% started in the initial phase. However, the literature indicates that the treatment of recurrent episodes of cold sores should begin at the prodromal stage and no more than 48 hours from the beginning of the lesions to achieve the best results.32
In the present study, most of the patients used the topical acyclovir drug, whose time ranged from 1 to 3 days (26.4%), 3 to 7 days (34.6%), and more than 7 days (34.6%), with an average of five applications per day.
During the study, 22 patients had a recurrence of herpes cycle and the results were similar to the first cycle of herpes. Most started treatment in the closed vesicle stage (50%). Signs of improvement were observed after the second (45.5%) and third applications (36.4%), and the disappearance of the symptoms was after 2 days to 45.5% and from 2 to 3 days to 45.5%. It is suggested that for the first cycle and the relapse cycle, the rapid signs of improvement were stated by the patients due to the anesthetic effect of the new formulation that eliminates the symptoms: pain, discomfort, burning, and burning sensation.
In addition, local anesthetics are also given anti-inflammatory, antimicrobial, and antiviral action.20-23 In 1972, Poste and Reeve33 conducted an initial study investigating the effects of various local anesthetics (dibucaine, tetracaine, cocaine, lidocaine, and procaine) on HVS-induced bovine kidney cell fusion and the authors reported that all local anesthetic agents induced a significant inhibition of cell fusion and proposed that local anesthetics exert this inhibition by occupying sites within the plasma membrane fusion occurs.
Cassuto23 evaluated treatment with lidocaine/prilocaine at the beginning of prodromal symptoms and suggested that the inhibitory effects of topical local anesthetics on herpes simplex involve mechanisms such as the interruption of the local axonal reflexes in the skin involved in the inflammatory response associated with the activation of the virus or by interference in viral membrane interactions interfering with virus penetration. Kutchai and Geddis34 suggested the antiviral effects of local anesthetics, as these agents have the ability to inhibit membrane ATPase, as well as the release of lysozyme and free radicals. In view of this, it can be suggested that the low healing time of the lesions (1-3 days) found in this study is due to the undefined antiviral activity of topical anesthetics.
Based on the results, it can be concluded that after the use of the new formulation, there was an effective decrease in the signs and symptoms of the herpetic lesions of the labial and peri buccal lesions, with dosage convenience (only three applications in a 24-hour period) and a low production cost, with a much shorter healing time than that reported using topical antiretrovirals.
Regarding psychosocial influences when compared to conventional treatments, the new treatment improved the participants' relationship, due to the reduction of discomfort, pain, and/or irritation; reduced the exposure time of the active lesions in the social environment, and finally, allowed the reduction of the time of treatment and permanence of the lesion in up to 72 hours, minimizing the occurrences of incomplete treatment, which is easily observed in prolonged treatments.
In addition, virus carriers also have the option of performing the entire drug application protocol at times of lesser social contact. The present product allowed to treat herpes easily, with easy applications by the participants and the product could treat the lesions with promisor results on all the four symptomatic phases of the herpes simplex 1, excluding the healing phase.
The proposed new treatment promoted a psychosocial improvement and well-being of the participants, against a pathogeny so inconstant and with peculiar characteristics. The great acceptance of the new product adds fundamental values for adherence to the treatment with better effectiveness and avoids the stigma of the color of the reference cream of the market, which highlights the lesion in some cases.
ACKNOWLEDGMENTS
The authors would like to thank Prof Freitas and Dr Ferreira for their support to make feasible this study. The authors also wish to thank Prof Pedrazzi for the effort to make this product available for this study. The authors wants to thank for the grant from the Sao Paulo Research Foundation (FAPESP) on the present study on 2012/07251-6 and 2017/24356-0 grants.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
MDRB was the head of the study, responsible for the figures and main structure of the present study. FATdeF was responsible for the analysis of the results, interpretations, and the main writing of the present study. APM, ACFS, and MPF were responsible for the sampling and experimental phase for the study. OdeF and VP were the head of the laboratories where all the experiments were made and were responsible for this study's achievements. VP was responsible for the idealization of the present work.




