Epidemiology and persistence of cervical human papillomavirus infection among outpatient women in Heilongjiang province: A retrospective cohort study
Abstract
As persistent carcinogenic human papillomavirus (HPV) infection is a prominent driver of cervical cancer, it is essential to explore HPV persistence and its associated factors for cancer screening and prevention. A retrospective cohort study was performed in outpatient women between March 2010 and 2019 in Heilongjiang, northeast China. HPV genotyping was performed by polymerase chain reaction-membrane hybridization. An unconditional logistic regression model was used to analyze the association of factors with persistence. The overall prevalence of HPV at baseline was 27.1%, with a downward trend from 2010 to 2019 (P < .0001). The most commonly observed high- and low-risk HPVs were HPV16 (N = 1094, 5.9%) and HPV11 (N = 596, 3.2%), respectively. The probabilities of 6-month persistence were high for women infected with HPV16 (P = .0001), HPV58 (P = .018), and HPV53 (P = .014), as well as for women with multiple infections (P = .009), and those who were 51 to 60 years old (P = .004) or more than 60 years old (P = .007). The probabilities of 12-month persistence were high for women infected with HPV53 (P = .017) and 51- to 60-year-old women (P = .044). HPV16 is the dominant HPV type in Heilongjiang. An age in the range of 51 to 60 years and infection with HPV53 is associated with HPV infection persistence in the Heilongjiang population.
Highlights
-
A retrospective study to assess HPV prevalence and persistence in cervix.
-
HPV prevalence is on a downward trend, with an overall prevalence of 27.1%.
-
HPV16 is the most prevalent type but not the most persistent type.
-
Age 51-60 years and HPV 53 infection are risk factors for persistent infection.
1 BACKGROUND
Human papillomavirus (HPV) is a major causal factor of cervical cancer, the fourth most common malignancy in women.1 Over 200 HPVs have been identified.2 Based on their relationship with malignant neoplasms, the mucosal HPV types are divided into low-risk (Lr) and high-risk (Hr) HPVs. Lr-HPVs (types 6, 11, 13, 32, etc,) are mainly induced benign lesions.3 However, 12 types (HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, and 59) are the etiological agents of cancer, and International Agency for Research on Cancer classified these HPVs as Hr-HPVs.2 A 2012 study of cervical cancer indicated that 90% of patients with cervical cancer were carrying HPV type 6, 11, 16, 18, 31, 33, 45, 52, and/or 58, with 71% of patients carrying types 16 and 18 globally, which are considered to be particularly carcinogenic.4 HPV exposure is very high, with some 80% of sexually active women acquiring an HPV infection during their lifetimes.5 Fortunately, most HPV infections clear spontaneously within 12 months of detection, and only a small portion of infections lead to cervical dysplasia or cancer.6 The progression of cervical cancer has been associated with several risk factors, namely infection with an oncogenic HPV genotype, a high viral load, having sexual intercourse before 21 years of age, multiple infections, smoking, and a high number of sex partners.7-10
HPV prevalence and distribution of HPV types have been shown to vary by country and across geographic regions.2, 7, 11, 12 Although HPV vaccination has been shown to prevent HPV infection,13 all carcinogenic types are not fully covered by currently available vaccines, such as the nine-valent vaccine, which targets HPV6/11/16/18/31/33/45/52/58.4 There remains a need to better understand HPV epidemiology and persistence to inform the development of national cervical cancer vaccination strategies, thereby improve cervical cancer-related public health with HPV screening and prophylactic vaccination.
Studies of HPV persistence have been variable concerning the definitions of persistent infection applied, testing intervals, HPV detection methods, and statistical analysis methods.7, 9, 14 Researchers in the Netherlands and Brazil6, 8 have used virus clearance rates and clearance duration times to assess HPV persistence. To the best of our knowledge, type-specific HPV persistence in Heilongjiang, the most northeastern province in China, has not yet been examined, it would be interesting to describe it in China.
The purpose of this study was to investigate the epidemiology and persistence of HPV infection in outpatient women in Heilongjiang. In addition, we examined the contributions of factors previously implicated in HPV persistence, including age, HPV type, and multiple infections. The data produced by this study are intended to be useful for HPV vaccination programs, with particular relevance for programs in northeastern China.
2 MATERIALS AND METHODS
2.1 Study design and participants
A large retrospective study was performed between March 2010 and 2019 in the Department of Obstetrics and Gynecology at the Fourth Affiliated Hospital of Harbin Medical University in Heilongjiang, northeast China. It was conducted in compliance with the Declaration of Helsinki, and the study protocol was approved by the Committee of Medical Science Ethics of the Fourth Affiliated Hospital of Harbin Medical University. Women living in Heilongjiang province who underwent cervical HPV testing as part of their routine gynecological evaluations were enrolled, the exclusion criteria were: being pregnant, history of hysterectomy, and any carcinoma diagnosis. Cervical HPV testing refers to HPV genotyping. As long as women are not excluded from the criteria, they can be included in the study regardless of their level of cytological testing. All the women included have the same ethnic background, representing the population of Heilongjiang, northeast China.
A flowchart of the screening subjects is presented in Figure 1. A total of 18 522 women were included in our initial epidemiological survey. Of 5011 women (27.1% of those surveyed) who were HPV-positive women at baseline, 1022 (20.4%) had a follow-up visit with repeat testing, 878 of whom had valid return visits. Follow-ups were considered valid for persistence analysis if they occurred ≥1 month after the baseline visit. Following the exclusion of 313 of these women for insufficient return visits, the remaining 565 women were screened rigorously and thus included in our HPV persistence analysis.
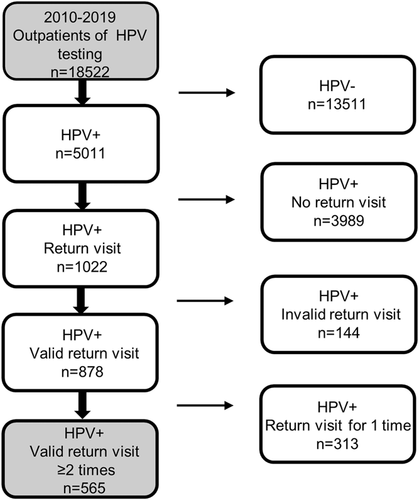
As is common in epidemiologic studies,15 6 and 12 months type-specific persistence were defined rigorously as a positive test at baseline followed by at least two positive tests for the same HPV type over a 6 and 12 months type-specific persistence, respectively. Type-specific HPV clearance was defined as a positive test followed by at least two consecutive negative tests for the same HPV type16; two type-specific HPV-positive results with one intermittent negative result were thus considered to be consistent with persistence. In this study, HPV infection persistence was analyzed relative to baseline, infection recurrence after clearance was not considered to be persistent.
2.2 Cervical specimen collection and DNA extraction
To optimize DNA analysis accuracy, the subjects were required not use any intravaginal medications and to refrain from sex for 48 hours, and we did not perform acetic acid testing. Cervical cell samples were collected with disposable cervical exfoliated cells samplers (Hybribio Biotechnology Limited Corp, Chaozhou, China). The sampler was inserted deep into the cervical canal and rotated gently for three to five turns, then placed in a sample preservation tube containing a cell preservation solution and placed in refrigerated sample storage (4°C) immediately. Cervical exfoliated cells were shaken, centrifuged at 14 000 rpm for 1 minute; the supernatant was discarded. DNA was extracted from exfoliated cells with lysis kits (Hybribio Biotechnology Limited Corp,) according to the manufacturer's instructions, and stored at −20°C.
2.3 HPV genotyping
HPV genotyping was performed by polymerase chain reaction (PCR)-membrane hybridization with a 21 HPV GenoArray diagnostic kit containing 13 Hr-HPVs (types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68), 5 Lr-HPVs (types 6, 11, 42, 43, and 44), and additional HPVs that are relatively common in China (types 53, 66, and CP8304). PCRs were conducted in a thermal cycler (KP-TC48), and an automated nucleic acid molecule rapid hybridization instrument (HBHM-3000S) was applied for HPV typing. Each PCR was conducted with 1 μL of DNA template according to the following cycling conditions: preheating at 95°C for 9 minutes, 40 cycles of denaturation at 95°C for 20 seconds, annealing at 55°C for 30 seconds, extension at 72°C for 30 seconds, and a final extension at 72°C for 5 minutes. The GenoArray Kit, thermal cycler, and automated hybridization instrument kit, required for HPV testing were all purchased from Hybribio Biotechnology Limited Corp. The amplification products were hybridized with membrane-bound type-specific oligonucleotides, and visible hybridization signals signified the presence of particular HPV genotypes.
2.4 Statistical analysis
Statistical analyses of persistence were based on type-specific HPV infections at baseline rather than on individual subjects, taking into account the occurrence of multi-HPV infection obviating the need to exclude other types of HPV infection. Potential associations of the following factors with HPV persistence were analyzed: age, multiple infections, and baseline infection with HPV16, HPV58, HPV52, HPV53, and HPV39.
All analyses were performed in the SAS software version 9.4 (SAS Institute Inc, Cary, NC). The baseline characteristics of the study population were analyzed and presented as frequencies and as means with standard deviations (SDs). Infection rates were compared with χ2 or Fisher's exact tests. Odds ratios (ORs) are reported with 95% confidence intervals (CIs). Trend analysis was carried out with Cochran-Mantel-Haensze tests. Unconditional logistic regression analysis was used to probe associations of independent variables with persistence. P values less than .05 were considered statistically significant.
3 RESULTS
3.1 Study participants
The epidemiological survey cohort of 18 522 women had a mean age of 38.8 years (SD: 10.3, range: 18-89 years). Of the 565 women included in our persistence analysis, 334 had two follow-up visits, 124 had three, 70 had four, and 37 had more than four follow-up visits. The mean number of return visits was 2.7 (SD: 1.13, range: 2-11), with a median time interval of 3.53 months.
3.2 HPV prevalence
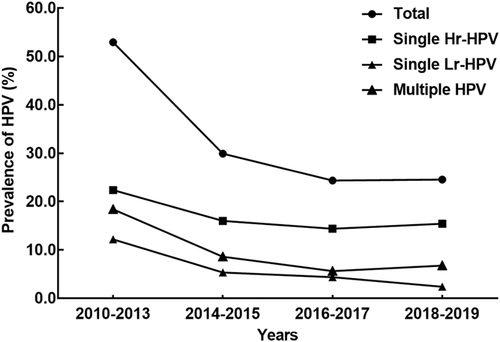
Trends in HPV prevalence over time across different infection groups (all, single Hr, single Lr, and multiple HPV infections) are shown in Figure 2. The highest HPV prevalence rates were observed in the 2010 to 2013 time period, in which there was a 52.9% HPV-positive rate overall, a 22.4% single Hr-HPV-positive rate, an 18.3% multiple-type HPV-positive rate, and a 12.1% single Lr-HPV-positive rate, respectively. Trend testing indicated a declining HPV prevalence over time in all infection groups (P < .0001). The overall, single Hr, single Lr, and multiple-type HPV-positive rates for the whole cohort and by age band are reported in Table 1. Single-type infections were more prevalent than multiple-type infections (P < .0001), and among single infections, Hr types were more common than Lr types (P < .0001). Concerning the age, participants in the 18 to 30 years age band had the highest infection rate overall, as well as within the single Lr-type and multiple-type infection groups (P < .0001 vs other age bands). Trend tests showed no significant effects of age on single Hr-HPV infection rates (P = .267) but showed downward trends with increasing age for single Lr-type infection and multiple-type infection (both P < .0001).
| Single infections | ||||
|---|---|---|---|---|
| Age, y | Hr-HPV n (%) | Lr-HPV n (%) | Multiple infections n (%) | Positive infections n/Total (%) |
| 18-30 | 731 (15.5) | 305 (6.5) | 505 (10.7) | 1541/4712 (32.7) |
| 31-40 | 886 (15.1) | 216 (3.7) | 326 (5.6) | 1428/5874 (24.3) |
| 41-50 | 813 (15.1) | 217 (4.0) | 301 (5.6) | 1331/5397 (24.7) |
| 51-60 | 342 (16.2) | 83 (3.9) | 158 (7.5) | 583/2113 (27.6) |
| >60 | 79 (18.5) | 11 (2.6) | 38 (8.9) | 128/426 (30.1) |
| Total | 2851 (15.4) | 832 (4.5) | 1328 (7.2) | 5011/18 522 (27.1) |
- Abbreviations: HPV, human papillomavirus; Hr-HPV, high-risk HPV genotype infection; Lr-HPV, low-risk HPV genotype infection.
3.3 HPV genotype distribution
The 21 HPV genotypes tested in this study were all detected (Figure 3). As shown in Figure 3A, among the Hr-HPV-type infections detected, the most frequent type was HPV16 (N = 1094, 5.9%; P < .001), followed by HPV58 (N = 655, 3.5%), HPV52 (N = 498, 2.7%), HPV39 (N = 472, 2.6%), and HPV53 (N = 461, 2.5%). Meanwhile, among the Lr-HPV-type infections detected, the most frequent types were HPV11 (N = 596, 3.2%), HPV6 (N = 490, 2.7%), and HPV CP8304 (N = 342, 1.9%), with low detection rates for HPV43 (N = 38, 0.2%), HPV44 (N = 37, 0.2%), and HPV42 (N = 18, 0.1%). Similarly, in participants with single or multiple HPV-type infections, HPV16, HPV58, HPV39, HPV52, and HPV53 were the top five Hr genotypes, while HPV6, HPV11, and HPV CP8304 were the most common Lr genotypes (Figure 3C,D).
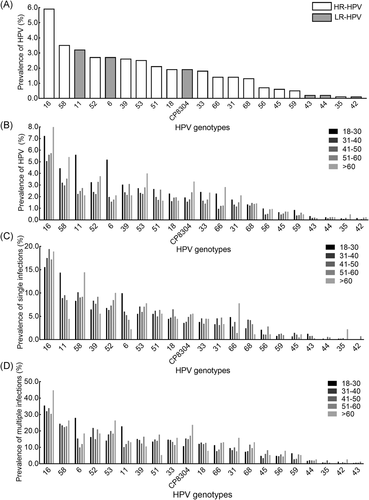
Analysis of HPV genotype distribution by age and type (Figure 3) showed that the HPV16 infection rate was significantly higher in the youngest (18-30 years) and oldest (>60 years) groups of women compared with the three other age groups (P < .0001). A similar pattern was observed for HPV58 (P = .002). HPV52, 39, 51, 33, 56, 59, and 66 infection rates also differed significantly among the age groups (P < .05). The infection rates for HPV6 and HPV11 were significantly higher in the 18 to 30 years age group than in the other age groups (P < .0001). As shown in Figure 3C,D, separate analyses of single-infection type cases and multiple HPV-type infections showed no significant differences among age groups for the five HPV types with the highest infection rates (ie, HPV16, 58, 52, 39, and 53).
3.4 HPV persistence
Percentages of type-specific HPV infections persisting for 6 and 12 months are shown in Table 2. The persistence analyses included 565 participants carrying a total of 738 type-specific infections (585 Hr-HPV and 153 Lr-HPV). Type-specific HPV persistence rates were higher at 6 months (30.9%) than at 12 months (22.1%; P = .002). Hr-HPV persistence rates were higher than Lr-HPV persistence rates for both periods (P < .05). We did not observe any persistent infections with HPV35 or HPV59 (Hr types) nor with HPV43 or HPV44 (Lr types).
| 6 mo Persistent infection | 12 mo Persistent infection | ||||
|---|---|---|---|---|---|
| HPV genotype | N | % (95% CI) | HPV genotype | N | % (95% CI) |
| Hr-HPV | 198/585 | 33.9 (30.0-37.7) | Hr-HPV | 71/291 | 24.4 (19.4-29.4) |
| 66 | 10/20 | 50 (27.2-72.8) | 58 | 12/30 | 40 (22.7-59.4) |
| 58 | 29/62 | 46.8 (34.0-59.9) | 53 | 6/15 | 40 (16.3-67.7) |
| 53 | 16/36 | 44.4 (27.9-61.9) | 52 | 9/26 | 34.6 (17.2-55.7) |
| 16 | 56/131 | 42.8 (34.2-51.7) | 16 | 18/72 | 25 (15.5-36.6) |
| 52 | 23/59 | 39 (26.6-52.6) | 39 | 5/22 | 22.7 (7.8-45.4) |
| 31 | 9/27 | 33.3 (16.5-54.0) | 68 | 4/19 | 21.1 (6.05-45.6) |
| 45 | 3/11 | 27.3 (6.0-61.0) | 66 | 2/10 | 20 (2.5-55.6) |
| 56 | 4/16 | 25 (7.3-52.4) | 56 | 2/10 | 20 (2.52-55.6) |
| 39 | 14/57 | 24.6 (14.1-37.8) | 18 | 4/22 | 18.2 (5.2-40.3) |
| 68 | 8/34 | 23.5 (10.8-41.2) | 33 | 3/18 | 16.7 (3.58-41.4) |
| 51 | 10/44 | 22.7 (11.5-37.8) | 51 | 4/25 | 16 (4.5-36.1) |
| 33 | 7/33 | 21.2 (9.0-38.9) | 31 | 2/14 | 14.3 (1.8-42.8) |
| 18 | 9/45 | 20 (9.6-34.6) | 35 | 0/1 | 0 |
| 35 | 0/1 | 0 | 45 | 0/5 | 0 |
| 59 | 0/9 | 0 | 59 | 0/2 | 0 |
| Lr-HPV | 30/153 | 19.6 (13.2-26.0) | Lr-HPV | 10/75 | 13.3 (5.5-21.2) |
| CP8304 | 11/42 | 26.2 (13.9-42.0) | CP8304 | 4/22 | 18.2 (5.2-40.3) |
| 42 | 1/4 | 25 (0.6-80.6) | 11 | 4/29 | 13.8 (3.9-31.7) |
| 11 | 11/58 | 19 (9.9-31.4) | 6 | 2/19 | 10.5 (1.3-33.1) |
| 6 | 7/42 | 16.7 (7.0-31.4) | 42 | 0/2 | 0 |
| 43 | 0/5 | 0 | 43 | 0/1 | 0 |
| 44 | 0/2 | 0 | 44 | 0/2 | 0 |
| Total | 228/738 | 30.9 (27.6-34.2) | Total | 81/366 | 22.1 (17.9-26.4) |
- Abbreviations: CI, confidence interval; HPV, human papillomavirus; Hr-HPV, high-risk HPV genotype infection; Lr-HPV, low-risk HPV genotype infection.
In the 12 months period persistence analysis, HPV58 and HPV53 were the most persistent types (both 40.0%), followed by HPV52 (34.6%), HPV16 (25%), and HPV39 (22.7%). Notably, HPV66 (1.4% prevalence) had a high type-specific persistent infection rate at month 6 (50.0%), whereas HPV18 (1.9% prevalence) has a relatively low persistence at month 6 (20.0%). The five most prevalent Hr-HPV types (HPV16, 58, 53, 52, and 39) were highly persistent at 6 and 12 months, though the most concerning Hr-HPV type, HPV16, was not the most persistent type.
3.5 Factors that influence persistence
The results (ORs, 95% CIs, and P values) of our analysis of potential persistence-influencing factors (age, multiple infections, and infection with each of the five most prevalent viral genotypes, namely HPV16, 58, 39, 52, and 53) on HPV persistence at 6 and 12 months are reported in Table 3. Multivariable analysis showed that HPV persistence at the 6-month time point was associated with the following cofactors: infection with HPV16, HPV58, or HPV53; being in one of the two oldest age bands (51-60 and >60 years); and multiple infections are cofactors influencing 6 months persistence. It is worth noting that women who were more than 60 years old were found to have 18.7 times increased risk of persistence than women in the 18 to 30 years age band. Among women with infections that persisted for 12 months, those infected with HPV53 had an almost fourfold increased risk of 12 months persistence relative to women infected with other HPV types, and women who were in the 51 to 60 years age band continued to have an increased risk of HPV persistence compared with women in the 18 to 30 years age band.
| 6 mo Presistent infection | 12 mo Presistent infection | |||
|---|---|---|---|---|
| Covariate | OR (95%CI) | P value | OR (95%CI) | P value |
| HPV16 | ||||
| Yes | 2.4 (1.6-3.8) | .0001 | 1.1 (0.6-2.2) | .692 |
| No (reference) | 1 | 1 | ||
| HPV58 | ||||
| Yes | 2.1 (1.1-3.7) | .018 | 2.2 (0.9-5.3) | .063 |
| No (reference) | 1 | 1 | ||
| HPV52 | ||||
| Yes | 1.5 (0.8-2.8) | .161 | 2.1 (0.9-5.1) | .097 |
| No (reference) | 1 | 1 | ||
| HPV39 | ||||
| Yes | 1.1 (0.6-2.2) | .611 | 1.3 (0.4-3.6) | .587 |
| No (reference) | 1 | 1 | ||
| HPV53 | ||||
| Yes | 2.6 (1.2-5.5) | .014 | 3.9 (1.3-12.8) | .017 |
| No (reference) | 1 | 1 | ||
| Age, y | ||||
| 18-30 (reference) | 1 | 1 | ||
| 31-40 | 0.8 (0.5-1.4) | .451 | 1.5 (0.6-4.0) | .581 |
| 41-50 | 1.0 (0.6-1.6) | .937 | 1.1 (0.4-3.1) | .579 |
| 51-60 | 2.5 (1.3-4.8) | .004 | 2.6 (1.0-7.2) | .044 |
| >60 | 18.7 (3.1-359.7) | .007 | 6.5 (1.5-25.0) | .489 |
| Multiple infection | ||||
| Yes | 1.8 (1.2-2.9) | .009 | 1.8 (0.9-3.4) | .19 |
| No (reference) | 1 | 1 | ||
- Note: The statistically significant P values were highlighted in bold.
- Abbreviations: CI, confidence interval; HPV, human papillomavirus; OR, odds ratio.
4 DISCUSSION
A retrospective cohort study was performed to investigate HPV epidemiology, HPV persistence, and factors associated with HPV infection persistence in outpatient women in Heilongjiang province, China. The total prevalence of HPV infection found in Heilongjiang in this study (27.1%) was similar to rates reported for southwestern China (26.2%)17 and central southern China (26.5%)18 but somewhat higher than rates reported for the provinces of Zhejiang (22.8%), Guangdong (20.16%), and Fujian (20.57%).19-21 Substantially lower rates have been reported for the city of Shenzhen (13.8%),22 the Xinjiang territory (14.02%),23 the city of Foshan (13.5%),24 and the city of Wuhan (17.68%).25 These differences in HPV infection rates may be related to different methods, including the use of different virus detection assays and the use of hospital-based versus population-based study cohorts. We also found that the youngest age band of women in our study (18-30 years) had a higher HPV infection rate than the other age bands in Heilongjiang, a finding that is consistent with HPV infection data from Xinjiang, Zhejiang, Wuhan, and Foshan.19, 23-25 HPV infection was associated with some sexual practices,26 so this age difference may be related to the sexual activity of young women.
Notably, we documented an apparent decline in HPV prevalence over time in all infection groups in Heilongjiang. Given the age effect mentioned above (women aged 18-30 years had a higher HPV infection rate than the other age bands), as well as a higher portion of 18 to 30 year women in 2010 to 2013 than in the subsequent time periods (P < .0001, data not shown), we considered that variations in the age compositions of tested women over time might lead to a decline in HPV prevalence. It also should be noted that the level of knowledge about HPV and its role in cancer development among Chinese women was very low in the past.27, 28 At present, in Heilongjiang, the number of tested women has increased every period. Therefore, this decline may reflect, at least in part, the rising awareness of cervical cancer among Chinese women.
Although there is some interregional variance regarding the relative prevalence rates of HPV genotypes in China, the genotypes that we found to be most common in Heilongjiang (HPV16, HPV58, and HPV52) are also common in most other examined parts of China.17, 19, 22-25 We treated HPV53 and HPV66, which are particularly common HPV genotypes in China, as Hr-HPVs, as others have done,17, 29 because cases of cervical cancer in patients with single HPV53 and single HPV66 infections have been reported, although rarely.30
In this study, we tested for a large number of HPV types and then focused our analysis of HPV persistence on those types with high infection rates. A consensus has yet to be established regarding how long it should be used to define HPV persistence. However, most studies have applied a minimum HPV persistence duration in the range of 6 to 12 months.15 We elected to monitor HPV infection persistence because persistence for more than a year is more likely to progress into cervical intraepithelial neoplasia or cervical cancer.15 In the analysis of HPV persistence, we found that being in the 51 to 60 years age band and being infected with HPV53 were associated with a significantly greater likelihood of HPV infection persistence.
Interestingly, HPV16—the main highly prevalent carcinogenic type of HPV—did not have a particularly high persistence rate and was not a risk factor for 12 months persistence. Nevertheless, in a study conducted in the Netherlands, Schmeink et al7 reported that HPV types 45, 31, 16, and 18 were most likely to persist for 12 months (60.0%, 56.8%, 54.4%, and 50.0%, respectively) and that having multiple HPV infections was positively associated with persistence at 12 months. HPV18 was also reported to be the most persistent type of HPV infection in Columbian women.9 In Heilongjiang province, however, we did not observe prominent persistence of HPV18, whereas being older emerged as an important factor related to HPV infection persistence. Our study differed from prior studies methodologically, most notably concerning the particular types of HPV subjected to persistence analysis and the definitions (time intervals) of persistence used. Different research methods may underlie, at least in part, differences in the results obtained.
HPV detection based on exfoliated cervical cell analysis is often insufficient to support secondary experiments. Because conventional PCR approaches do not enable HPV genotype identification, we utilized PCR-membrane hybridization technology, which has been shown previously to be highly reliable, sensitive, and accurate.24, 25, 31 We chose this method to minimize false-negative results and provide clinically relevant reference values.
In addition, this study has some limitations. Because it was a retrospective study, it was not feasible to analyze more factors concerning HPV infection persistence, such as the number of sexual partners, thin-prep cytologic test results, education level, smoking, and alcohol intake. This study included only 565 participants in the final analysis, and larger samples should be examined in future studies of HPV infection persistence.
In conclusion, the present population-based screening cohort study provides detailed epidemiological data for infections with 21 HPV types diagnosed between 2010 and 2019 in Heilongjiang province in China. Epidemiological investigations of HPV infection have thus far been regional in China, with no definitive report on cervical HPV infection prevalence rates across China having been produced to date. To the best of our knowledge, this has been the first study to analyze the prevalence and persistence of type-specific HPVs in Heilongjiang. These findings may be used to inform the development of vaccines targeting the Heilongjiang region. Given that not all persistent infections cause cervical cancer, it will be necessary for future studies to examine the mechanism of persistence of major carcinogenic HPV types and to identify biomarkers related to persistent infection. It would be useful to differentiate participants according to the thin-prep cytology level as well as cervical lesion status to analyze how these factors might be related to HPV type-specific persistence and cervical cancer risk.
ACKNOWLEDGMENTS
This study was supported by the National Natural Science Foundation of China (Grant no. 81672670), Heilongjiang Province Outstanding Youth Foundation (JC2018023).
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
LW conceived and designed the study. JW, SH, LW were involved in data collection and analysis. DF and YS performed the genotyping assays. JL drafted the manuscript. All authors have read the final version of the manuscript upon which they agreed.
ETHICS STATEMENT
This study was conducted under compliance with the Declaration of Helsinki and was approved by the Committee of Medical Science Ethics of the Fourth Affiliated Hospital of Harbin Medical University.




