The distribution of hepatitis B virus surface antigen polymorphisms at positions associated with vaccine escape
Abstract
Hepatitis B virus (HBV) infects over 250 million people worldwide. Vaccination is effective at preventing infection, although several mutations within the “a” determinant region of the HBV surface antigen (HBsAg) are associated with vaccine escape. We evaluated the frequency, genotype, and global distribution of polymorphisms at sites associated with vaccine escape in 4244 unique full-length HBV genomes. The “a” determinant within the Surface gene was inspected for polymorphisms at sites identified previously associated with vaccine escape. Nearly, 268 (6.3%) sequences from 36 countries contained a polymorphism at a site associated with vaccine escape including 22 genotype A, 99 genotype B, 93 genotype C, 32 genotype D, 14 genotype E, 3 genotype F, 2 genotype G, and 3 genotype I. In genotype A, the most common polymorphism occurred at M133. In genotype B, Q129 and M133 occurred 45 and 51 times, respectively, accounting for 94% of polymorphisms. Polymorphisms at G145 were most frequent in genotype C, while P120 was most common in genotype D. Among all genotypes, polymorphisms at M133 were the most common and accounted for 30.9% of polymorphisms. Polymorphisms at T116, P120, F134, K141, and P142 occurred in geographically diverse locations, whereas polymorphisms at Q129, M133, D144, and G145 were concentrated in East Asia. While the sample size is large, this approach relied on convenience sampling within each country, and many countries have no data available, thereby highlighting the need for additional routine surveillance of surface antigen mutations associated with vaccine escape.
Highlights
-
Mutations within the ‘a’ determinant region of the hepatitis B virus (HBV) surface antigen (HBsAg) are associated with vaccine escape.
-
We evaluated the frequency of polymorphisms at sites associated with vaccine escape in over 4,000 unique full-length HBV genomes.
-
268 (6.3%) sequences from 36 countries contained a polymorphism at a site associated with vaccine escape.
-
Routine surveillance of surface antigen mutations associated with vaccine escape is needed in many countries.
1 INTRODUCTION
Hepatitis B virus (HBV) infects over 250 million people and is the leading cause of hepatitis and hepatocellular carcinoma worldwide.1 Vaccination is considered the single best preventative measure against HBV infection, and the World Health Organization recommends that all children receive their first vaccine dose as soon as possible, preferably within 24 hours of birth. By the end of 2018, HBV vaccination for infants had been introduced nationwide in 189 countries. In addition, 109 countries administered one dose of HBV vaccine to newborns within the first 24 hours of life, although the global coverage was only 42%.1
The HBV vaccine induces antibodies specific to the “a” determinant region of the HBV surface antigen (HBsAg), which is comprised of amino acids (aa) 124 to 147. Amino acid substitutions within the “a” determinant region can lead to vaccine escape. For instance, the first vaccine escape mutation—a glycine (G) to arginine (R) substitution at position 145 within the “a” determinant that caused conformational changes that permitted the virus to escape the vaccine-induced immune response—was reported in 1988 postexposure in an Italian child who received passive-active immunization at birth.2, 3 Since the discovery of G145, additional escape mutations have been identified, both in the setting of vertical transmission of HBV from mothers to their infants and de novo mutations in vaccinated persons.4-6 Furthermore, additional mutations within the “a” determinant region, such as Q129N, M133T, and F134Y, may facilitate diagnostic failure of HBsAg detection.7
Complete genome analysis suggests the existence of at least eight HBV genotypes (A-H).8, 9 HBV genotype may influence chronicity, disease severity, and antiviral response rates. Moreover, the mutation rate is relatively high due to the low fidelity of the viral reverse transcriptase.10 Within a single individual, HBV exists as a population of highly related, yet distinct, viral variants termed the viral quasispecies that facilitates rapid, adaptive changes in response to immune selection pressures and antiviral therapy.11 Polymorphisms at vaccine escape-associated sites may arise for several reasons, including (1) natural variation within the viral genome, (2) immune (antibody or cell-mediated) selection pressures, (3) recombination, and/or (4) drug resistance (ie resistance-associated mutations within the polymerase open reading frame that also result in an amino acid change within the surface open reading frame).
While some researchers have recently called for systematic surveillance of HBV mutations associated with vaccine escape,12, 13 large population-based studies of HBV vaccine escape and vaccine escape mutations are uncommon due to cost, the high prevalence of HBV in many resource-limited settings, and the relatively rare outcome of HBV infection in vaccinated individuals. As no systematic data exist on the prevalence of HBV surface antigen mutations in diverse geographic locations, we evaluated the distribution of amino acid polymorphisms at known vaccine escape sites by genotype and geographic location from a large database of HBV sequences.
2 METHODS
Full-length HBV genome references were downloaded from http://hvdr.bioinf.wits.ac.za/alignments/index.html.14 The HVDR data set—and not partial sequences from NCBI—was selected for several reasons, including (1) the HVDR data set has been curated extensively and includes a large number of full-length sequences, (2) the subsequently analysis of polymorphisms reported here requires extensive sequence alignment that would not be practical with larger but less complete data sets, (3) partial NCBI sequences have the added challenge of not being rigorously genotyped in many instances due to the smaller amount of sequence data available. The HVDR data set included 4244 unique sequences including 508 from genotype A, 1000 from genotype B, 1543 from genotype C, 727 from genotype D, 230 from genotype E, 170 from genotype F, 20 from genotype G, 17 from genotype H, and 29 from genotype I. Six references were excluded. Five references—JF032342, JF032344, JF032352, JF032353, and JF032354—were removed subsequently from PubMed, and one reference—FJ348219—was labeled as human mitochondrial DNA. The “a” determinant region within S ORF (aa 124 to 147) was extracted from all sequences using AliView version 1.23 and visually inspected for polymorphisms at previously identified vaccine escape sites including T116, P120, T126, Q129, M133, F134, K141, P142, D144, or G145.2, 7, 15-21 For each reference with at least one such polymorphism, the accession number was used to determine the country of origin and the year of sample collection. Full-length sequences were entered into Geno2Pheno at https://www.geno2pheno.org/ to confirm HBV genotype, subgenotype, and the presence of vaccine escape mutations. Weblogos were created for each genotype A through F using the site https://weblogo.berkeley.edu/logo.cgi but were not generated for genotypes G, H, or I due to the limited number of sequences available. Weblogos represent frequency plots with the relative height of each amino acid (only positions previously associated with vaccine escape are shown) presenting its proportion for that genotype. Microsoft Excel was used to generate maps highlighting the location of polymorphisms at vaccine escape-associated positions that were identified within each country. Due to the large sample numbers, China and Malaysia are shown separately. A schematic of the overall workflow is included in Figure S1.
3 RESULTS
In total, there were 310 polymorphisms at vaccine escape-associated sites with some sequences containing multiple polymorphisms (Table 1). Thus, 268 unique sequences from 36 countries contained a polymorphism at a vaccine escape site, accounting for 6.3% of the total data set of 4244 sequences. The 268 unique sequences included 22 from genotype A, 99 genotype B, 93 genotype C, 32 genotype D, 14 genotype E, 3 genotype F, 2 genotype G, and 3 genotype I. In genotype A, the most common polymorphism occurred at M133, accounting for 8 of 29 polymorphisms. Of 102 polymorphisms in genotype B, polymorphisms at Q129 and M133 occurred 45 and 51 times, respectively, accounting for 94% of polymorphisms. Polymorphisms at G145 accounted for 36 of 116 in genotype C, followed by M133 with 28. P120 accounted for 18 of 41 polymorphisms in genotype D. Of all 268 sequences, polymorphisms at M133 were the most common and accounted for 96 of 310 (30.9%).
| Genotype (N) | T116N | P120E | T126ANIS | Q129HR | M133L | F134ILT | K141E | P142S | D144AE | G145RA | Total (% of total) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A (508) | 2 | 1 | 3 | 1 | 8 | 4 | 3 | 2 | 3 | 2 | 29 (9.4) |
| B (1,000) | 4 | 45 | 51 | 2 | 102 (32.9) | ||||||
| C (1,543) | 2 | 7 | 18 | 7 | 28 | 5 | 2 | 11 | 36 | 116 (37.4) | |
| D (727) | 1 | 18 | 2 | 6 | 4 | 5 | 1 | 2 | 2 | 41 (13.2) | |
| E (230) | 1 | 2 | 2 | 5 | 3 | 1 | 14 (4.5) | ||||
| F (170) | 2 | 1 | 3 (1.0) | ||||||||
| G (20) | 1 | 1 | 2 (0.6) | ||||||||
| I (29) | 1 | 2 | 3 (1.0) | ||||||||
| Total | 6 | 32 | 25 | 62 | 96 | 9 | 9 | 6 | 19 | 46 | 310 |
Figure 1 shows the frequency of specific amino acid substitutions at known escape mutation sites for genotypes A to F. For the entire data set that included all 4244 sequences from genotypes A to I, the most common polymorphisms found were T116N, P120S, T126I, Q129H, M133L, F134Y, K141E/I, P142L, D144V, and G145R (Figure 2). However, specific genotypes differed in the most prevalent amino acid substitution at individual sites associated with vaccine escape, while other genotypes did not contain any polymorphisms at certain escape-associated positions. For genotype A, the most common polymorphisms were T116N, P120R, T126I, Q129R, M133I/T, F134L, K141T/I/E, D144A, and G145S/A. For genotype B, the most common polymorphisms were P120S, Q129H, M133L, and G145R/A. There were no genotype B sequences with polymorphisms at T116, T126, F134, K141, P142, or D144. For genotype C, the most common polymorphisms were T116A/P, P120T, T126S, Q129R, M133T, K141E/I, P142L, D144V, and G145R. There were no genotype C sequences with polymorphisms at F134. For genotype D, the polymorphisms identified were T116N, P120S, T126I, Q129R, M133T, F134N/S, P142L, D144E/G, and G145R, but there were no polymorphisms at position K141. For genotype E, T116N, P120L/S, Q129H/R, M133I, D144E, and G145I were the most common polymorphisms, and the genotype E sequences examined contained no polymorphisms at T126, F134, K141, and P142. Among the most common polymorphisms for genotype F were T126A/P and Q129L. Genotype F did not contain polymorphisms at any vaccine escape-associated sites.
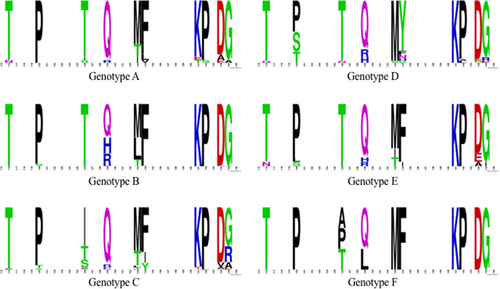
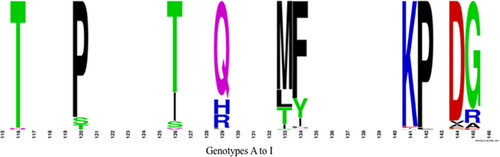
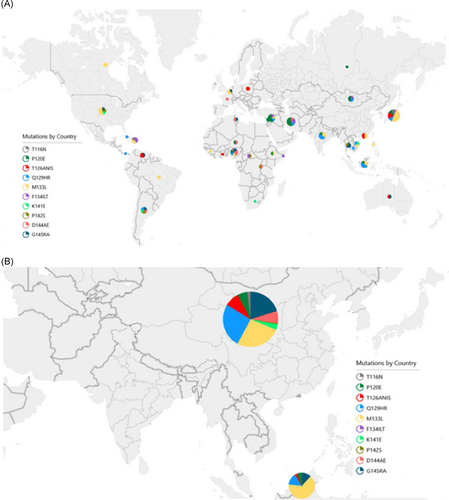
Table 2 includes the number of sequences with polymorphisms at known vaccine escape sites by country of origin. The specific polymorphisms observed within each country are also shown in Figure 3. These data suggest that polymorphisms at positions T116, P120, F134, K141, and P142 occur in geographically diverse locations, whereas polymorphisms at positions Q129, M133, D144, and G145 are concentrated in East Asia. The countries contributing the largest number of polymorphisms—China (155) and Malaysia (34)—are also in this region. The most prevalent polymorphism in the Middle East and North Africa (Iran, Lebanon, Tunisia, and Syria) was at position P120—representing 11 of 20 polymorphisms from the region—whereas M133 was most prevalent in North America and Europe (Canada, France, Belgium, Poland, Russia, and United States) accounting for 8 of 16 polymorphisms. Q129 accounted for 3 of 7 polymorphisms in India, D144 for 4 of 18 polymorphisms in Africa (CAR, Ethiopia, Ghana, Guinea, Niger, Nigeria, Rwanda, Somalia, and South Africa), and M133 for 4 of 18 polymorphisms in South and Central America (Argentina, Brazil, Cuba, Haiti, Panama, and Venezuela).
| Country | T116N | P120E | T126ANIS | Q129HR | M133L | F134ILT | K141E | P142S | D144AE | G145RA | Total (% of total) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Argentina | 1 | 1 | 1 | 1 | 1 | 5 (1.6) | |||||
| Australia | 1 | 1 | 2 (0.6) | ||||||||
| Belgium | 2 | 1 | 3 (1.0) | ||||||||
| Brazil | 2 | 2 (0.6) | |||||||||
| Cambodia | 1 | 1 (0.3) | |||||||||
| Canada | 2 | 2 (0.6) | |||||||||
| CAR | 1 | 1 | 2 (0.6) | ||||||||
| China | 3 | 9 | 13 | 40 | 41 | 5 | 1 | 11 | 32 | 155 (50.0) | |
| Cuba | 1 | 1 (0.3) | |||||||||
| Ethiopia | 1 | 1 | 2 (0.6) | ||||||||
| France | 1 | 1 (0.3) | |||||||||
| Ghana | 1 | 1 (0.3) | |||||||||
| Guinea | 1 | 1 (0.3) | |||||||||
| Haiti | 1 | 2 | 2 | 5 (1.6) | |||||||
| Hong Kong | 2 | 2 | 4 (1.3) | ||||||||
| India | 1 | 3 | 2 | 1 | 7 (2.3) | ||||||
| Indonesia | 1 | 3 | 2 | 6 (1.9) | |||||||
| Iran | 5 | 1 | 2 | 1 | 9 (2.9) | ||||||
| Japan | 1 | 2 | 3 | 2 | 9 | 1 | 18 (5.8) | ||||
| Lebanon | 2 | 2 (0.6) | |||||||||
| Malaysia | 2 | 1 | 5 | 22 | 4 | 34 (11.0) | |||||
| Mongolia | 2 | 1 | 1 | 4 (1.3) | |||||||
| Niger | 1 | 1 | 2 (0.6) | ||||||||
| Nigeria | 1 | 1 | 1 | 1 | 2 | 1 | 7 (2.3) | ||||
| Panama | 1 | 1 (0.3) | |||||||||
| Philippines | 1 | 1 (0.3) | |||||||||
| Poland | 2 | 2 (0.6) | |||||||||
| Russia | 1 | 1 (0.3) | |||||||||
| Rwanda | 1 | 1 | 2 (0.6) | ||||||||
| Somalia | 1 | 1 (0.3) | |||||||||
| South Africa | 1 | 1 (0.3) | |||||||||
| Syria | 4 | 1 | 1 | 1 | 7 (2.3) | ||||||
| Thailand | 1 | 2 | 3 (1.0) | ||||||||
| Tunisia | 1 | 1 | 2 (0.6) | ||||||||
| United States | 4 | 1 | 1 | 1 | 7 (2.3) | ||||||
| Venezuela | 2 | 1 | 3 (1.0) | ||||||||
| Vietnam | 1 | 1 | 1 | 3 (1.0) | |||||||
| Total | 6 | 32 | 25 | 62 | 96 | 9 | 9 | 6 | 19 | 46 | 310 |
Of the 268 sequences with polymorphisms at the amino acids of interest, 39 (14.6%) contained at least two mutations, including 6 of 22 (27.3%) genotype A sequences, 2 of 99 (2.0%) genotype B sequences, 23 of 93 (24.7%) genotype C sequences, and 8 of 32 (25.0%) genotype D sequences.
4 DISCUSSION
Population-based studies of HBV vaccine failure and the emergence of vaccine escape mutations are rare and have not been conducted in most countries. Nonetheless, a study conducted in Taiwan among HBsAg-positive children before and after the implementation of a nationwide vaccination program found that 8 of 103 (7.8%) in 1984, 9 of 46 (19.6%) in 1989, and 5 of 20 (25%) in 1994 harbored at least one mutation within the “a” determinant region.16 The authors concluded that universal vaccination may have accelerated the accumulation of HBsAg mutations critical for immune escape in vaccinated children. Subsequent studies in the same setting reported mutations within the “a” determinant peaked at 28.1% in 1994 and remained at 23.1% and 22.6% in 1999 and 2004, respectively.17, 22 A study conducted in China in the postvaccination era found that “a” determinant mutations were relatively stable over time but that levels of G145R/A mutations increased with time.23 A French study compared the prevalence of “a” determinant mutations between two groups of chronic hepatitis B patients—one group that was persistently HBsAg positive/anti-HBs positive and another that was solely HBsAg positive. The number of Surface gene mutations was 2.7 times more frequent in the HBsAg positive/anti-HBs group, with most of the changes occurring at positions G145, Q129, T126, and D144.24 Although there is concern that mutations within the “a” could undermine the vaccination efforts, the prevalence of escape mutants has remained stable over time in at least one setting.22 Data from blood donors suggest that strains carrying “a” determinant mutations may be more commonly associated with occult HBV infections and/or low-level viremia.25 As well, data from a prospective study conducted in India suggest that while HBV vaccination with or without administration of hepatitis B immunoglobulin prevents transmission of chronic HBV from HBsAg-positive mothers to their infants, it may not prevent occult (HBsAg negative but HBV DNA positive) infections in exposed infants.26
Using a large global data set, the current study found that 6.3% of published HBV sequences contained at least one polymorphism at a position associated with vaccine escape. The genotypic distribution of this sample set reflected those found in other epidemiological studies of HBV.27 Table 3 summarizes the number of genotypes by country of origin. For instance, East Asia featured predominantly genotypes B and C, whereas the Middle East, India, and Central Asia contained mostly genotype D. Sub-Saharan and East Africa contained samples from genotypes D and E, whereas Southern Africa contained mostly genotype A. Most sequences from Europe and North America belonged to genotype A. Finally, South America and Central America included genotypes A, D, F, and G.
| Country | A | B | C | D | E | F | G | I | Total (% of total) |
|---|---|---|---|---|---|---|---|---|---|
| Argentina | 2 | 1 | 3 (1.1) | ||||||
| Australia | 2 | 2 (0.7) | |||||||
| Belgium | 1 | 2 | 3 (1.1) | ||||||
| Brazil | 2 | 2 (0.7) | |||||||
| Cambodia | 1 | 1 (0.7) | |||||||
| Canada | 2 | 2 (1.1) | |||||||
| Central African Republic | 1 | 1 (0.4) | |||||||
| China | 59 | 73 | 4 | 2 | 138 (51.5) | ||||
| Cuba | 1 | 1 (0.4) | |||||||
| Ethiopia | 1 | 1 | 2 (0.7) | ||||||
| France | 1 | 1 (0.4) | |||||||
| Ghana | 1 | 1 (0.4) | |||||||
| Guinea | 1 | 1 (0.4) | |||||||
| Haiti | 3 | 3 (1.1) | |||||||
| Hong Kong | 2 | 2 (0.7) | |||||||
| India | 1 | 6 | 1 | 8 (3.0) | |||||
| Indonesia | 6 | 6 (2.2) | |||||||
| Iran | 5 | 5 (1.9) | |||||||
| Japan | 4 | 5 | 4 | 13 (4.8) | |||||
| Lebanon | 2 | 2 (0.7) | |||||||
| Malaysia | 22 | 8 | 30 (11.2) | ||||||
| Mongolia | 2 | 2 (0.7) | |||||||
| Niger | 2 | 2 (0.7) | |||||||
| Nigeria | 7 | 7 (2.6) | |||||||
| Panama | 1 | 1 (0.4) | |||||||
| Philippines | 1 | 1 (0.4) | |||||||
| Poland | 2 | 2 (0.7) | |||||||
| Russia | 1 | 1 (0.4) | |||||||
| Rwanda | 1 | 1 (0.4) | |||||||
| Somalia | 1 | 1 (0.4) | |||||||
| South Africa | 1 | 1 (0.4) | |||||||
| Syria | 6 | 6 (2.2) | |||||||
| Thailand | 2 | 2 (0.7) | |||||||
| Tunisia | 1 | 1 (0.4) | |||||||
| United States | 5 | 1 | 1 | 7 (2.6) | |||||
| Venezuela | 2 | 1 | 3 (1.1) | ||||||
| Vietnam | 2 | 1 | 3 (1.1) | ||||||
| Total | 22 | 99 | 93 | 32 | 14 | 3 | 2 | 3 | 268 |
This global evaluation shows the frequency and geographic distribution of polymorphisms at known vaccine escape positions by genotype. Escape mutations are frequently suspected when HBV DNA is detected in previously vaccinated subjects. PCR and DNA sequencing is then employed to identify mutations within the “a” determinant region of these infected patients. In cases where vaccinated HBV-positive infants are born to HBV-positive mothers, sequencing of HBV from both mother and child can detect changes in the “a” determinant region that occurred after transmission, lending evidence that certain mutations arise after pressure from a vaccinated host's immune system. These mutations can be characterized further by testing their binding affinity using commercially available monoclonal and polyclonal anti-HBs. For instance, the mutations Q129N, M133T, F134L, and G145R have been associated with negative results in diagnostic tests using monoclonal and polyclonal antibodies against HBsAg,7 whereas one study reported the P120Q, D144A, and G145R mutations in fully vaccinated children with detectable HBV DNA and HBeAg positivity.16 Other mutations, such as T116A, have yet to be characterized functionally for either vaccine escape or low antigenicity in tests using HBsAb.
Several limitations to this study should be considered. First, while the sample size is quite large, this is not a true prevalence study. The data set relied on other researchers to generate the sequence data and to make it publicly available. The HBV sampling strategy within each country is typically not weighted based on the relative prevalence of HBV in that country, and the distribution of genotypes may not be representative. This can be seen most readily in two countries—China and Malaysia—that accounted for a large proportion of the available sequence data set. Second, HBV sequences were available from only 37 of over 190 countries, and many countries provided no data at all for analysis. Third, the year of sample collection was not uniform and did not reflect the initiation of widespread vaccination campaigns in many countries (Figure S2); thus, any change in the prevalence of vaccine escape-associated polymorphisms over time was not possible. Fourth, in many studies, the anti-HBs antibody status and/or receipt of a full vaccine dose/course were not reported; therefore, it is not possible to determine, which individuals completed a full HBV vaccination schedule or to make conclusive statements regarding the presence of vaccine escape mutations within individuals that were vaccinated. Fifth, in many instances, the surface antigen mutations have been proven to cause clinically significant infection in successfully vaccinated but HBV exposed children and adults. However, this is not the case for some mutations that represent polymorphisms at sites associated with vaccine escape but have not been evaluated in vitro, nor among individuals with a documented immunologic response to HBV vaccination. Ultimately, whether HBV vaccination truly prevents infection with mutant virus can only be evaluated in chimpanzee challenge experiments. Sixth, the sociodemographic data that could be abstracted from each accession number varied significantly by country. For example, data on the sex, race, serological markers, and infection status of the individuals from whom samples were obtained were frequently excluded from accession numbers. Finally, full-length genome sequences from the HVDR data set were evaluated, although partial viral sequences are also available. As much of this partial sequence data has not been analyzed rigorously and/or may not be genotyped accurately, we focused on well-characterized full-length HBV sequences. Despite these potential limitations, the prevalence of escape mutations is high and may compromise efforts to control HBV infection. These data should be considered carefully by policy makers when evaluating the overall efficacy of current vaccination strategies.
ACKNOWLEDGMENTS
MR was funded by the Internal Medical Scholarly Training for Academic Research (IM STAR) program at the University of Cincinnati College of Medicine. WTC was supported through the Sub-Saharan African Network for TB/HIV Research Excellence (SANTHE), a DELTAS Africa Initiative [grant # DEL-15-006]. The DELTAS Africa Initiative is an independent funding scheme of the African Academy of Sciences (AAS)'s Alliance for Accelerating Excellence in Science in Africa (AESA) and supported by the New Partnership for Africa's Development Planning and Coordinating Agency (NEPAD Agency) with funding from the Wellcome Trust (grant #107752/Z/15/Z) and the UK government. The views expressed in this publication are those of the author(s) and not necessarily those of AAS, NEPAD Agency, Wellcome Trust, or the UK government.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
MH and JTB designed the study. MH and WTC conducted the data analysis. MH drafted the initial manuscript. MH, WTC, and JTB edited the final manuscript.