A lesson learnt from the emergence of Zika virus: What flaviviruses can trigger Guillain-Barré syndrome?
Abstract
While Zika virus outbreaks raised the concern about rare viral infections in human, attention should also be paid to other Guillain-Barré syndrome (GBS) inducing viruses. This study aims to search for other flaviviruses likely to be associated with GBS. Amino acid (aa) sequence matching analysis was conducted to identify viruses molecularly similar to the Zika virus and human GBS-related proteins. A systematic review of clinical literature was performed to summarize the clinical findings of the GBS-associated flaviviruses identified in the aa sequence matching analysis. It was found that more than 10 pentapeptides were shared between 9 flaviviruses, Zika virus, and human GBS-related proteins. Twenty-six articles totaling 42 clinical cases were eligible for inclusion in the systematic review concerning the nine flaviviruses identified. While some patients showed signs of encephalitis, 5 out of 42 cases demonstrated typical GBS symptoms. Public health professionals should be aware of other GBS-associated flaviviruses and GBS cases with mild symptoms.
1 INTRODUCTION
While the human infection was first detected in 1952, Zika virus (ZIKV) was not widely known to the public until 2007 during the sudden epidemic in Micronesia, followed by the outbreaks in Oceania in 2013 and Americas in 2015. Accompanying the growing number of ZIKV infections was the high incidence of Guillain-Barré syndrome (GBS).1 Given the strong association between GBS and viruses of the Flaviviridae family, global warming is likely to increase the infection rates of mosquito-borne diseases as well as GBS.
Clinically characterized by the elevated level of protein in the cerebrospinal fluid (CSF), GBS is a postinfectious peripheral polyneuropathy consisting of a group of rare and acute immune-mediated disorders of the peripheral nervous system. Most common symptoms include weakness, numbness, and tingling in extremities, followed by rapidly progressive paralysis. As such, GBS is one of the most common causes of neuromuscular respiratory failure with 30% patients requiring mechanical ventilation,2 and early and prompt diagnosis is needed. In the absence of sufficiently sensitive biomarkers,3 differential diagnosis is usually adopted with the use of CSF analysis, nerve conduction study, and electromyography.
The global annual incidence of GBS is reported to be 0.6 to 2.4 cases per 100 000 per year4 and 0.34 to 1.34 for aged 18 or below.5 The incubation period of GBS follows the Sartwell model with a median of 13 to 14 days, counting from the antecedent infection.6 Campylobacter jejuni is the most common agent associated with GBS.7 A number of flaviviruses have been reported to be associated with GBS, including Dengue virus, Japanese encephalitis virus, West Nile virus,8 St. Louis encephalitis virus,9 Tick-borne encephalitis virus,10 yellow fever virus (through attenuated vaccine11), and most recently ZIKV.
A large body of research has been done to investigate the pathogenesis of GBS with molecular mimicry being commonly hypothesized to be the cause of GBS.12 First noted when a protein of herpes simples virus type 1 was found to cross-react with an intermediate filament protein of human cells,13 molecular mimicry refers to the sharing of a linear amino acid (aa) sequence between a microbe and a host. Autoimmune diseases are generated when antibodies are stimulated to react against the host. In the context of GBS, antibodies attack peripheral nerve sites, leading to neural damages. While studies concerning the pathogenesis largely focus on the characterization of the gangliosides and antiganglioside antibodies, research on identifying GBS-associated viruses is useful from an epidemiological perspective.
This paper aims to raise the awareness of the outbreak of GBS by finding other possible GBS-inducing flaviviruses and by providing a systematic review of the human infection cases of these viruses. This paper proceeds as follows. The next section provides a description of the methodologies used—aa sequence matching analysis and a systematic review, followed by the results and discussion.
2 METHODS
2.1 Amino acid sequence matching analysis
To identify possible GBS-associated flaviviruses, the aa sequence matching methodology developed in existing literature was adopted.14, 15 Under the hypothesis of molecular mimicry, common peptides must be shared between GBS-related human proteins and the polyprotein of the GBS-associated virus. The aa sequence matching analysis was conducted using the pentapeptide as a minimal immune unit as it can induce highly specific antibodies.14 A match was considered found in the event of an exact pentapeptide being shared between two proteins.
Previous research15 has identified a total of 222 pentapeptides shared between ZIKV and the set of human GBS-related proteins. A hundred and thirty-five of the 222 pentapeptides were then found to be shared with the proteins of some GBS-associated pathogens including influenza A virus (H5N1 and H1N1), human papillomavirus 16, hepatitis E virus genotype I, Epstein-Barr virus, varicella-zoster, dengue virus, West Nile virus, yellow fever virus, mycoplasma pneumoniae, and C. jejuni. These 135 pentapeptides were used to further search for other possible GBS-inducing flaviviruses. UniProt, a freely accessible protein sequence database, and the PIR peptide match program (https://research.bioinformatics.udel.edu/peptidematch/index.jsp) were used for the matching analysis.
2.2 Systematic review
To seek signs of possible flavivirus-induced GBS, the present work provided a systematic review of clinical cases associated with the possible GBS-inducing zoonotic flaviviruses identified in the aa sequence matching analysis. Given that a large amount of viruses may be identified in the aa sequence matching analysis, below 10 flaviviruses were studied.
The systematic review was conducted in line with PRISMA. The search strategy involved the use of PubMed, LILAC, and Embase for clinical case identification with the virus name as the search term, for example, “Ilheus virus” for Ilheus virus. To enhance efficiency, irrelevant articles were avoided by searching with the exact name of the virus. For instance, the search term “Ilheus virus” could rule out articles concerning “Ilheus” as the city in Brazil. Articles in English, French, and Spanish were considered. There was no restriction on the date of publication. In the screening process, some articles in the search results were excluded after title analysis and duplicates were removed. Inclusion criteria include, (a) confirmed cases, (b) clear description of symptoms, and (c) diagnostic tests. Exclusion criteria include (a) articles not describing individual cases and (b) articles the authors had no access to (such as those not available electronically and out-of-print). The following data were abstracted, (a) location, (b) age, (c) gender, (d) diagnostic tests, (e) protein in CSF, if available, (f) GBS-related symptoms, and (g) flavivirus-related symptoms.
3 RESULTS
A total of 49 960 viruses were included in UniProt at the time of analysis. The proteomes of 388 viruses (excluding subtypes and different strains, as defined by UniProt) entailed at least one of the 135 pentapeptides. Some of those associated with GBS were found to share with more than five pentapeptides (Figure 1). A number of flaviviruses were found to entail more than 10 pentapeptides (Table 1). After excluding those reportedly to be associated with GBS and those not pathogenic to human, nine flaviviruses were found to be possible viruses inducing GBS. They were Ilheus virus (ILHV), Rocio virus (ROCV), Usutu virus (USUV), Kokobera virus (KOKV), Bussuquara virus (BSQV), Murray valley encephalitis virus (MVEV), Banzi virus (BANV), Edge Hill virus (EHV), and Wesselsbron virus (WSLV).
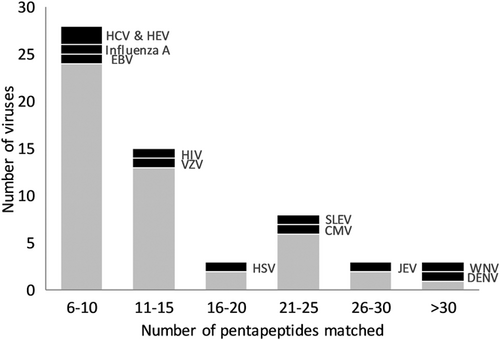
| Virus | Number of pentapeptides matched | Family | Remarks |
|---|---|---|---|
| Zika virus | 135 | Flaviviridae | Reportedly GBS associated |
| Dengue virus | 41 | Flaviviridae | Reportedly GBS associated |
| Kunjin virus | 34 | Flaviviridae | Subtype of West Nile virus |
| West Nile virus | 33 | Flaviviridae | Reportedly GBS associated |
| Acanthamoeba polyphaga mimivirus | 29 | Mimiviridae | Nonhuman pathogen |
| Ilheus virus | 29 | Flaviviridae | |
| Equine herpesvirus | 28 | Herpesviridae | |
| Japanese encephalitis virus | 28 | Flaviviridae | Reportedly GBS associated |
| Enterobacteria phage lamda | 27 | Siphoviridae | Nonhuman pathogen |
| Human cytomegalovirus | 25 | Herpesviridae | Reportedly GBS associated |
| Rocio virus | 25 | Flaviviridae | |
| Usutu virus | 24 | Flaviviridae | |
| Kokobera virus | 23 | Flaviviridae | |
| St. Louis encephalitis virus | 23 | Flaviviridae | Reportedly GBS associated |
| Bussuquara virus | 22 | Flaviviridae | |
| Murray valley encephalitis virus | 22 | Flaviviridae | |
| Human herpes simplex virus | 19 | Herpesviridae | Reportedly GBS associated |
| Invertebrate iridescent virus | 19 | Iridoviridae | Nonhuman pathogen |
| Escherichia phage (AR1, lamda, Mu, N15, P2, T5) | 17 | Multiple | |
| Banzi virus | 15 | Flaviviridae | |
| Human herpesvirus 6 | 15 | Herpesviridae | |
| African swine fever virus | 14 | Asfarviridae | Nonhuman pathogen |
| Psittacid herpesvirus | 14 | Herpesviridae | Nonhuman pathogen |
| Varicella-zoster virus | 13 | Herpesviridae | Reportedly GBS associated |
| Ictalurid herpesvirus | 12 | Alloherpesviridae | Nonhuman pathogen |
| Tick-borne encephalitis virus | 12 | Flaviviridae | Reportedly GBS associated |
| Yellow fever virus | 12 | Flaviviridae | Reportedly GBS associated |
| Edge Hill virus | 11 | Flaviviridae | |
| Gallid herpesvirus | 11 | Herpesviridae | Nonhuman pathogen |
| Human adenovirus | 11 | Adenoviridae | |
| Human immunodeficiency virus | 11 | Retroviridae | Reportedly GBS associated |
| Human papillomavirus | 11 | Papillomaviridae | |
| Suid herpesvirus | 11 | Herpesviridae | Nonhuman pathogen |
| Wesselsbron virus | 11 | Flaviviridae |
- Abbreviation: GBS, Guillain-Barré syndrome.
A total of 1437 articles were found in all three databases and 1267 articles were removed due to duplication and title analysis, as outlined in Figure 2. Most of the removed articles rather focused on the biological aspects of viruses such as genome characterization. After full-text assessments, 26 articles were included in the study, totaling 42 clinical cases (Table 2).
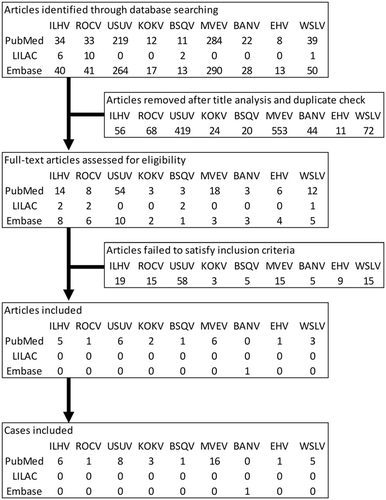
| Reference | Location | Age | Sex | Diagnosis tools | Protein in CSF, mg/dL | GBS-related symptoms | Symptoms |
|---|---|---|---|---|---|---|---|
| Ilheus virus | |||||||
| Johnson et al16 | Ecuador | 20 | M | 4,6 | Abdominal pain, epistaxis, fever, headache, jaundice, myalgia, nausea, rash, retro-ocular pain, sore throat, vomiting | ||
| Venegas et al17 | Bolivia | 15 | M | 5,6 | Abdominal pain, arthralgia, asthenia, bone pain, conjunctival injection, earache, facial edema, fever, headache, malaise, myalgias, vesicular rash | ||
| Spence et al18 | Trinidad | 37 | F | 2,3 | Hips paralysis | Constipation, diplopia, fever, headache, incessant cough, joint pain, lassitude, muscle pain, nausea, pain in lower limbs, photophobia, restlessness, signs of circulatory collapse, tachycardia | |
| Spence et al18 | Trinidad | 22 | M | 2,3 | Chills, fever, malaise | ||
| Prías-Landínez et al19 | Colombia | 28 | M | 1,2,3 | Bleeding | ||
| Srihongse and Johnson20 | Panama | N/A | M | 2,3 | Fever, headache | ||
| Rocio virus | |||||||
| de Souza Lopes et al21 | Brazil | 39 | M | 2,3 | Lower limb weakness | Drowsiness, fever, headache | |
| Usutu virus | |||||||
| Simonin et al22 | France | 39 | M | 4,6 | 67 | Peripheral facial palsy | Eyelid ptosis, paresthesias of both right limbs, prodromal dysgeusia, transient right upper limb palsy |
| Pecorari et al23 | Italy | 60s | F | 6 | Weakness at four limbs | Dysmetry, fever, tremor | |
| Aberle et al24 | Austria | N/A | N/A | 1,6 | Anorexia, chills, fever, headache, pain in joints, profuse sweating, restlessness | ||
| Nagy et al25 | Hungary | 40s | M | 4,5,6 | Choreiform movements, fever, internuclear ophthalmoplegia, irritability, seizure | ||
| Cavrini et al26 | Italy | 40s | F | 6 | Aphasis, confusion, drowsiness, fever, nausea | ||
| Santini et al27 | Croatia | 29 | F | 1,5 | 68 | Agitated, confusion, drowsy, fatigue, fever, hyoxemia, lethargy, tachypnea | |
| Santini et al27 | Croatia | 61 | M | 1,5 | 193 | Confusion, decreased consciousness, fever, headache, memory loss, neck stiffness, photophobia | |
| Santini et al27 | Croatia | 56 | M | 1,5 | 81 | Confusion, decreased consciousness, disorientation, fever, irritability | |
| Kokobera virus | |||||||
| Boughton et al28 | Australia | 40 | F | 2,5 | Altered consciousness, deficits in self-care, language and cognitive domain, dystonia, fever, seizures, spastic paraplegia | ||
| Boughton et al28 | Australia | 43 | M | 2,5 | Decreased consciousness, dizziness, fever, headache, left-right disorientation, lethargy, neck stiffness, short-term memory loss | ||
| Mein et al29 | Australia | 20 | M | 2,4 | Chills, fever, headache, myalgia, pharyngitis | ||
| Bussuquara virus | |||||||
| Srihongse and Johnson30 | Panama | 29 | M | 1,2,3 | Confusion, dysarthria, dysphasia, extrapyramidal gait isorder, fever, headachde, malaise, short-term memory loss, tremor, vomiting | ||
| Murray Valley encephalitis virus | |||||||
| Cordova et al31 | Australia | 10 mo | M | 2,4,5 | Disorientation, dysmetria, extensor plantar response, fever, headache, hyppereflexia, intention hand tremor, nausea, nuchal rigidity, somnolence, vomiting | ||
| Cordova et al31 | Australia | 55 | M | 4,5 | Chills, fever, headache, muscle spasm, nuchal rigidity | ||
| Cordova et al31 | Australia | 64 | M | 2,4,5 | Extensor plantar response, fever, headache, hyppereflexia, intention hand tremor, nuchal rigidity, somnolence, tongue tremor, transient diplopia | ||
| Cordova et al31 | Australia | 32 | M | 2,4,5 | Disorientation, fever, flaccid paralysis, headache, malaise, vomiting | ||
| Cordova et al31 | Australia | 25 | M | 2,5,6 | Deteriorating sensorium, fever, headache, neck stiffness | ||
| Cordova et al31 | Australia | 41 | F | 2,4,5 | Flaccid quadriplegia | Fever, seizures | |
| Cordova et al31 | Australia | 69 | M | 2,4,5 | Bulging anterior fontanelle, fever, flaccid paralysis below the neck, neck stiffness, seizures | ||
| Cordova et al31 | Australia | 61 | F | 4,6 | Fever, flaccid paralysis, headache | ||
| Cordova et al31 | Australia | 79 | F | 2,4,6 | Fever, headache, mild increment of cytolytic liver enzyme, rash | ||
| Niven et al32 | Canada | 19 | F | 5,6 | 33 then 92 | Fever, headache, nuchal rigidity | |
| Brown et al33 | Australia | 69 | M | 2,4,5 | 81 | Fever, headache, malaise, spastic paralysis | |
| Brown et al33 | Australia | 3 mo | F | 2,4,5,6 | 250 | Giddy, headache, vomiting | |
| Brown et al33 | Australia | 2 mo | M | 2,4,5 | Fever, irritability, seizure | ||
| French et al34 | Papua New Guinea | 14 | M | 1,2,3 | 25 | Headache, lethargy, pain in joints, pain in neck | |
| Smith et al35 | Australia | 18 mo | M | 2,4,5 | Normal level | Headache, pain in joints, pain in neck, rash, tiredness | |
| Floridis et al36 | Australia | 8 | M | 6 | 86 | Rash | |
| Banzi virus | |||||||
| Smithburn et al37 | South Africa | 9 | M | 1 | Fever | ||
| Edge Hill virus | |||||||
| Aaskov et al38 | Australia | 64 | M | 2 | Arthralgia, fatigue, myalgia | ||
| Wesselsbron virus | |||||||
| Jupp and Kemp39 | South Africa | 60 | M | 2,3,5 | Arthralgia, fever, headache, myalgia, rash | ||
| Heymann et al40 | South Africa | N/A | M | 1 | Anorexia, fever, headache, muscular and joint pains, pain behind eyes | ||
| Heymann et al40 | South Africa | N/A | M | 1 | Abdominal pain, fever, headache, muscular pain, pain behind eyes, rigor | ||
| Diagne et al41 | Senegal | 4 | F | 3,6 | Fever, headache, diffuse pain | ||
| Diagne et al41 | Senegal | 30 | F | 3,6 | Fever, jaundice | ||
- Note: 1 = Neutralization test, 2 = Haemagglutination inhibition, 3 = Complement fixation test, 4 = Immunofluorescence assay, 5 = ELISA, 6 = PCR.
- Abbreviations: CSF, cerebrospinal fluid; GBS, Guillain-Barré syndrome; PCR, polymerase chain reaction.
One-way analysis of variance and the Kruskal-Wallis test on age yielded a test statistic of 0.065 (P = .801) and 5.279 (P = .724) respectively, showing no significant age difference between the virus groups. χ2 homogeneity test statistic of 3.072 (P = .930) reveals no significant gender proportion difference between the nine flaviviruses. For geographical distribution, the cases of each virus were largely confined to a single continent with the exception of MVEV where the patient returning from Australia was diagnosed with MEVE infection in Canada.
For articles published between 1950s and 1970s, the complement fixation test remained a common diagnostic tool in those cases. While ELISA was a commonly used diagnostic tool in most cases, other tests were also performed due to cross-reactivity. For viruses with human infections rarely reported, commercial assays were not available and polymerase chain reaction (PCR) did not seem common in virus detection.
While symptoms were highly diverse, at least 75% of the cases in each virus were characterized by fever, except for EHV. Headache was another common symptom, characterizing at least 67% of the cases in each virus, with the exception for USUV. As flaviviruses might lead to encephalitis, lumbar puncture was conducted in 10 cases, accounting for 24% of all cases. Of these 10 cases, almost all had CSF protein above the reference range of 15 to 35 mg/dL. While the elevation of CSF protein was likely a result of encephalitis, peripheral neurologic symptoms were observed in a very few cases. GBS-related symptoms were observed in 5 out of 42 cases. There appeared some evidence suggesting the relationship between the number of pentapeptides matched and the proportion of cases with GBS-related symptoms. Given the violation of the normality assumption, Spearman's rank correlation coefficient between the two variables is estimated to be .7825, with P = .0127 suggesting significant correlation.
4 DISCUSSION
Similar to the epidemiologic status of ZIKV before a series of outbreaks, human infections of the flaviviruses studied in this paper are uncommon, either occasional cases reported from time to time or epidemics occurred a few decades ago. Public health professionals should be aware of the potential outbreaks of these viruses as well as possible GBS induced.
ILHV was first discovered in mosquitos in Ilheus City in Brazil in 1944. While no outbreak has ever been recorded, ILHV was widespread in much of northern South America as suggested by the high antibody rates in the population.20 However, clinical manifestations were not commonly seen. Cross-reactivity in serologic assays to other flaviviruses did occur, including ROCV, which is the most closely related virus.16 ILHV has been isolated from mosquitos of genera Ochlerotatus, Psorophora, Culex, Sabethes, Haemagogus, and Trichoprosopon.17 Reportedly, symptoms include mild accompanied by headache, myalgia, arthralgia, and photophobia.16
ROCV first appeared in an epidemic in Ribeira Valley of Brazil in the 1970s causing approximately 1000 diagnosed cases with about 100 deaths. With an incubation period of 7 to 14 days, symptoms include fever, headache, anorexia, nausea, vomiting, myalgia, and malaise.42 While the virus has been isolated from Psorophora ferox, little is known about ROCV including epidemiology as well as its appearance and disappearance in Ribeira Valley.42 Given serologic evidence of ROCV circulation, public health authorities were concerned about possible ROCV outbreaks in the future.42
First isolated from mosquitos in South Africa in 1959, first human infection of USUV was identified in 1981 in the Central African Republic. More recently, the first human infection outside Africa was detected in France in 2016.22 Because it is genetically related to West Nile virus and Japanese encephalitis virus,23 clinical manifestations are similar with infections being asymptomatic or occasionally associated with rash.24 USUV has widely circulated in Europe. Revealed by USUV-specific real-time PCR assays, USUV infections were identified in 18 out of 31 598 blood donations with most donors showing no symptoms.24
First isolated in 1960 from Culex annulirostris, KOKV is enzootic in Australia and Papua New Guinea.43 Clinical illness may resemble dengue while it is more often associated with arthralgia.29 Many aspects of KOKV remain not known as human infections are very rare.
Similar to KOKV, little is known about BSQV. Before its first isolation in Panama from human in 1971, BSQV was first discovered from a sentinel monkey in Brazil in 1956.30 Forty-six percent positivity in a serum survey of 383 samples suggested that BSQV was widespread among the population in certain areas in Panama.30 BSQV was also detected in human in a serosurveillance conducted in Colombia between 1956 and 1961.44 The virus has been isolated from mosquitos of the Culex genus.42
Since its first appearance as an epidemic in the late Summer of 1950 to 1951 in Eastern Australia,34 human infections of MVEV have been reported from time to time. While the majority of the infections were asymptomatic with between 1 in 150 and 1 in 1000 infection resulting in symptomatic disease,36 permanent neurological sequelae occurred in about half of the survivors.33 C. annulirostris is the major vector of MVE virus, Aedes normanensis is also considered to play a role in the transmission.33, 34
Only one single case of BANV and EHV infection has been reported,45 hence little is known about them. BANV was first isolated from a child in South Africa in 1956 and was also isolated from C. rebinotus.46 EHV was first isolated from a C. annulirostris in Australia in 1961. It was also isolated from other species such as A. Normanensis, A. Bancroftianus, A. Camptorhynchus, A. Vigilax in Australia between 1979 and 2000.47
WSLV was first isolated from infected sheep during an outbreak in South Africa in 1955. It also caused outbreaks in South Africa between 2010 and 2011,48 then in Senegal in 2013.41 Outside Africa, it has also been discovered once in Thailand.49 No fatal case has been reported so far.48 Human infections reportedly caused arthralgia, myalgia, and fever while neurological disease in horse have been observed.41 WSLV was isolated from A. mediolineatus and A. lineatopeninis.49
An immediate implication is that most of the abovementioned viruses are similar in clinical aspects. Their infections were asymptomatic or caused mild febrile illnesses. Serologic diagnosis can be difficult due to cross-reactive antibodies to other viruses. Immunological memory from previous flavivirus exposure may encumber the interpretation of antibody titers.
On the back of our finding of the significant correlation between the number of pentapeptide shared and the proportion of cases with GBS-related symptoms, possible GBS induced by flavivirus infection is another concern. The fact that GBS-related symptoms were seen in some of the reviewed cases and that patients were not treated for GBS suggested the possibility of mild GBS. While some patients with mild GBS were able to walk without assistance and might recover even left retreated, diagnosis is important as the prognosis of GBS remains unclear. Unfortunately, GBS and encephalitis can display similar clinical manifestations. Elevated CSF protein is commonly seen in patients with GBS or encephalitis. Moreover, normal levels of nerve conduction velocity may also be seen in patients with mild GBS.50 Medical professionals should be aware of possible mild GBS in patients infected with the flaviviruses identified in the present work.
Finally, there is a limitation concerning the findings in the present work. Similarity at the aa sequence does not provide casual evidence to conclude that the viruses identified can trigger GBS. Other criteria such as those involving animal models and epidemiological studies must also be fulfilled.