The role of M2-2 PDZ-binding motifs in pulmonary innate immune responses to human metapneumovirus
Abstract
Human metapneumovirus (HMPV) is a leading cause of lower respiratory tract infection (LRTI) in pediatric and geriatric populations. We recently found that two PDZ-binding motifs of the M2-2 protein, 29-DEMI-32 and 39-KEALSDGI-46, play a significant role in mediating HMPV immune evasion in airway epithelial cells (AECs). However, their role in the overall pulmonary responses to HMPV infection has not been investigated. In this study, we found that two recombinant HMPVs (rHMPV) lacking the individual M2-2 PDZ-binding motif are attenuated in mouse lungs. Mice infected with mutants produce more cytokines/chemokines in bronchoalveolar lavage (BAL) fluid compared to mice infected with wild-type rHMPV. In addition, both mutants are able to enhance the pulmonary recruitment of dendritic cells (DCs) and T cells and induce effective protections against the HMPV challenge. The DC maturation is also significantly improved by the motif mutation. Taken together, our data provide proof-of-principle for two live-attenuated M2-2 mutants to be promising HMPV vaccine candidates that are effective in inducing higher pulmonary innate immunity and generating protection against HMPV infection.
Highlights
-
HMPV is a major pathogen causing hospitalization in children with bronchiolitis and pneumonia. The infection does not induce long-lasting immunity. Herein, we identified two M2-2 mutants which are effective in inducing higher pulmonary innate immunity and producing protection against HMPV infection.
1 INTRODUCTION
Human metapneumovirus (HMPV) is a respiratory pathogen belonging to the Metapneumovirus genus in the Pneumoviridae family.1 It was first isolated in 2001 and was quickly recognized to be a major cause of lower respiratory tract infection (LRTI) in children, geriatric, and immunocompromised patients worldwide.2-4 Reinfection with HMPV occurs repeatedly throughout life without significant antigenic changes, suggesting the induction of insufficient or short-lived immunity.5 There are currently no effective vaccines or specific therapeutic agents available for individuals infected with HMPV.
To date, various HMPV vaccine candidates have been developed and tested in cell lines, animals, and humans. Among them, heat- or formalin-inactivated HMPV vaccines result in enhanced disease and higher mortality in immunized animals rather than unimmunized animals,6, 7 suggesting that these vaccines are not safe. Some vaccine candidates, such as subunit vaccines that consist of viral protein(s) in vectors or virus-like particles, do not result in enhanced disease in cotton rats and nonhuman primates after the induction of protective immunity. However, the immunity does not last long and multiple doses are needed to maintain antiviral immunity,8-10 suggesting the ineffectiveness of these candidates. On the other hand, some live-attenuated recombinant HMPV (rHMPV) lacking specific viral protein(s) have been shown as promising vaccine candidates, because they induce strong immunogenicity in animal models.11, 12 In addition, an rHMPV, which has an introduced mutation in SH to keep the overall HMPV genome stable, and a live-attenuated rHMPV with its P protein replaced with avian MPV P protein, have been shown as safe in immunized human volunteers,13, 14 which suggests that live-attenuated rHMPV could be a promising vaccine candidate. However, the molecular mechanisms underlying the attenuation of the live-attenuated rHMPV are largely unknown.
HMPV, with the genome length of 13Kb, encodes nine proteins: N, P, M, F, M2-1, M2-2, SH, G, and L. Some proteins, such as G, P, SH, and M2-2 have been shown to modulate host innate immunity to favor HMPV infection via different mechanisms.15-19 We have recently shown that the M2-2 protein of HMPV facilitates immune evasion by targeting the mitochondrial antiviral-signaling protein (MAVS) and myeloid differentiation primary response 88 (MyD88), two central antiviral signaling molecules in airway epithelial cells (AECs) and dendritic cells (DCs), respectively.18, 20 Furthermore, we identified that two putative PDZ-binding motifs of M2-2, 29-DEMI-32, and 39-KEALSDGI-46, contribute significantly to M2-2-mediated immune evasion in cells.21 However, their impacts on pulmonary responses to HMPV infection have not been explored.
To evaluate in vivo functions of these two motifs, we compared the virus replication, immune mediator induction, and immune cell recruitment/activation in the lungs between wild type (WT)-rHMPV-infected mice and mice infected with mutants lacking the individual motif. Overall, the data were consistent with our previous findings in the suppressive role of two M2-2 PDZ-binding motifs in cellular innate immunity.21, 22 Attenuated replication and enhanced host innate immune responses to mutant infections also suggested that rHMPV lacking either motif could serve as live-attenuated vaccine candidates.
2 MATERIALS AND METHODS
2.1 rHMPV preparation and titration
WT-rHMPV or recombinant mutants with mutations in the motif 29-DEMI-32 (Mut-1) or the motif 39-KEALSDGI-46 (Mut-2), were generated and propagated in LLC-MK2 cells (ATCC, Manassas, VA), as previously described.21, 23, 24 Viral titers of purified recombinant viruses or viruses from the infected mouse lungs were determined by immunostaining in LLC-MK2 cells, as described previously.21, 25
2.2 Mice
Six- to eight- week-old female BALB/c mice were purchased from The Jackson Laboratory and maintained under specific-pathogen-free conditions at the University of Texas Medical Branch (UTMB) at Galveston. To investigate the innate response of mice to the primary infection, mice were anesthetized and administered intranasally with 5 × 106 pfu of rHMPV, diluted in Dulbecco's PBS (D-PBS) (Invitrogen, Carlsbad, CA), or with an equivalent volume of sucrose diluted in D-PBS (mock-infection), as previously described.26 To study whether mice with primary inoculation of rHMPV develop protection, mice were challenged with 107 pfu of HMPV/CAN/97/83/A2 on day 28 postprimary infection. Lungs at day 5 postprimary infection or post-challenge were harvested to determine the HMPV titers in the lungs. All of the studies were approved by the UTMB Institutional Animal Care and Use Committee.
2.3 Bronchoalveolar lavage analysis
Bronchoalveolar lavage (BAL) fluid was collected by washing the lungs two times with 1 mL D-PBS at different days postinfection (p.i.). After centrifuging at 1500 rpm for 5 minutes at 4°C, the supernatants were used for quantification of cytokines/chemokines using Luminex-based Bio-Plex Pro Mouse Cytokine 23-plex Assay (Cat #M60009RDPD; Bio-Rad Laboratory, Hercules, CA) according to the manufacturer's instructions. The examined cytokines include Eotaxin, G-CSFm GM-CSF, interferon (IFN)-γ, interleukin (IL)-1α, IL-1β, IL-2, IL-3, Il-4, Il-5, IL-6, IL-9, IL-10, IL-12p40, IL-12p70, IL-13, IL-17A, KC, MCP-1, MIP-1α, MIP-1β, RANTES, and tumor necrosis factor-α. The detection limit for all cytokines by the Bioplex assay is 3 pg/mL. The IFN-β was quantified by enzyme-linked immunosorbent assay (ELISA) (PBL Biomedical Laboratories, Piscataway, NJ) with the detection limit of 50 pg/mL.
2.4 Airway hyperresponsiveness
Airway hyperresponsiveness (AHR) was assessed in unrestrained conscious mice by noninvasive nonventilated enhanced pause (Penh) measurement, as described previously.26, 27 Freely moving mice were placed in whole-body barometric plethysmography (Buxco Electronics, Troy, NY) and respiratory activity was recorded for 4 minutes to measure baseline Penh values.
2.5 Fluorescence-activated cell sorting analysis of immune cells
Lung samples of mice, mock-inoculated or inoculated with WT-rHMPV or mutants, were harvested at days 5 and 7 p.i. Total lung cells were prepared, as previously described.25, 26 Isolated cells were incubated with anti-FcγRII/RcγRIIImAb (24G2; BD Biosciences, San Jose, CA) followed by cell-surface marker staining. For cell-surface marker staining, isolated cells were stained with the following antibodies: anti-CD11c in combination with anti-CD80, anti-CD86, or anti-MHCII (all from BD Pharmingen, San Jose, CA) for DCs and anti-CD4 or anti-CD8 in combination with anti-CD3 (all from BD Pharmingen) for T cells. Samples were stained at 4°C in PBS with 1% fetal bovine serum and analyzed with a BD FACSCanto flow cytometer equipped with BD FACSDiva software (both from Becton Dickinson Immunocytometry Systems, Franklin Lakes, NJ). The analysis was performed using Cyflogic (Cyflo Ltd, Turku, Finland).
2.6 Statistical analysis
Statistical significance was determined by the Mann-Whitney U test. Values of P < .05 were considered significant. Values for viral replication, cell numbers, and cytokine/chemokine production experiments were presented as mean ± standard error (SEM) of the mean.
3 RESULTS
3.1 M2-2 PDZ-binding motifs are important to pulmonary HMPV replication and the modulation of pulmonary cytokine/chemokine induction
To determine whether the motifs 29-DEMI-32 and 39-KEALSDGI-46 are important in mediating pulmonary innate responses to HMPV, we first compared pulmonary viral loads in WT-, Mut-1-, or Mut-2-infected mice, at day 5 p.i., the replication peak of primary HMPV infection.26, 27 In brief, BALB/c mice were intranasally inoculated with WT, Mut-1, or Mut-2 at 5 × 106 pfu/mice, followed by lung harvesting and viral titration. The mock-inoculation was used as a negative control. As shown in Figure 1A, mice infected with Mut-1 and Mut-2 exhibited more than a log reduction in lung viral titer than those infected with WT, indicating the replication attenuation of mutants. To confirm the mutations after infection, we isolated total RNAs from infected lungs at day 5 p.i. The DNA sequencing of reverse transcription PCR products demonstrated that mutations of each motif were kept intact (data not shown), supporting the importance of motifs in the regulation of HMPV replication. The sequencing was done in the Molecular Genomics Core at UTMB.
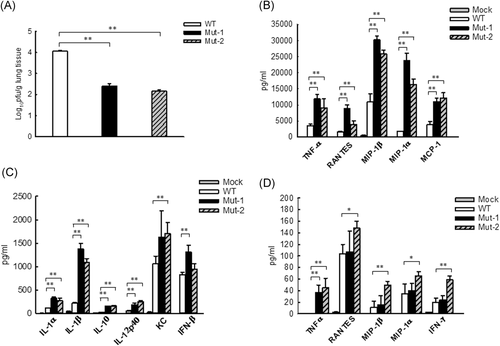
We then investigated the pulmonary cytokine/chemokine induction by infection, WT or mutants. In brief, we inoculated mice with WT or mutants, using mock-inoculation as a control, and measured the abundance of cytokines/chemokines in pulmonary BAL fluid at day 1 and 5 p.i by Bio-Plex Pro Mouse Cytokine 23-plex Assay (Bio-Rad). Generally speaking, the induction of most cytokines/chemokines by HMPV infection peaks at day 1 p.i., and goes back to the basal level at day 5 p.i.28 Herein, Figure 1B,C show the induction at day 1 p.i. Mediators with the induction >5000 pg/mL are listed in Figure 1B, while the mediators with the induction <2500 pg/mL are shown in Figure 1C. Compared with the cytokine/chemokine induction by WT-rHMPV, the induction of these molecules was significantly enhanced by Mut-1 or Mut-2 infection. Pulmonary IFN-β induction was comparable between WT- and Mut-2 infected lungs. At day 5 p.i., the abundance of pulmonary cytokines/chemokines became significantly less in all groups, compared with that at day 1 p.i. The ones detected are shown in Figure 1D. Our Bio-plex assay did not detect any induction of Th2 cytokines including IL-4, IL5, and IL-13 in all experimental groups. Overall, these results demonstrated that both motifs 29-DEMI-32 and 39-KEALSDGI-46 of M2-2 suppress the induction of pulmonary innate immune mediators after HMPV infection.
3.2 Mutant infection of mice provides protection against viral challenge with a clinical isolate
To investigate the impact of motifs on the lung function in response to HMPV infection, we compared the AHR in WT- and mutant-infected mice by Penh measurement, a method using a noninvasive and nonventilated unrestrained whole-body plethysmography to correlate changes in airway physiology by the infection.29-31 Mock-inoculation was used as a negative control. The basal Penh in response to HMPV infection usually peaks between day 5 and day 7 p.i.27 Therefore, we chose day 6 p.i. for the Penh investigation. We found that, at day 6 p.i., there were no significant Penh differences between WT- and Mut-2-infected mice, while Mut-1 induced moderately higher Penh than WT and Mut-2 (Figure 2A). At day 8 p.i., the Penh in response to WT, Mut-1, and Mut-2 infections became comparable among the groups.
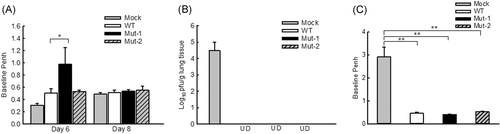
Innate inflammatory cytokines and viral loads are important determinants of lung pathogenesis. Both are generally suggested to be associated with the disease severity of HMPV and respiratory syncytial virus (RSV).32-40 Although the attenuation of mutant replication (Figure 1A) and enhanced induction of protective immune mediators, such as IL12-p40,33 could prevent severe lung injury from possible adverse effects of mutant-enhanced inflammatory cytokines, there are three recent publications from different groups, who supported that higher innate cytokines triggered by an essential amount of infectious particles are necessary to prevent severe RSV diseases.41-43 Viruses with a low inoculation indeed can cause more severe disease because of their failure to induce sufficient innate immune responses, demonstrating complicated balance mechanisms underlying disease severity.41-43 Although no such reports are available for HMPV infection, and we do not know whether mutant-enhanced cytokine/chemokine induction is beneficial or deleterious to lung functions, our Penh results did not show uncontrollable pathogenesis by mutant infections, compared with WT infection. No animal death was observed after the primary infection of WT or mutants either.
Next, to evaluate the protective capacity of rHMPV mutants, we challenged mice with HMPV/CAN/97/83/A2 at 107 pfu/mice on day 28 post-initial inoculation with WT or mutant rHMPV. The mock-inoculation was used as a negative control. The lungs were harvested at day 5 post-challenge for viral titration. No pulmonary viral particles could be detected in WT- and mutant rHMPV-inoculated mice, while mock-inoculated mice had significant virus replication (Figure 2B), suggesting that mutants could generate protection. We also did not observe the difference in Penh at day 5 post-challenge among mice which were immunized with WT, Mut-1, and Mut-2 (Figure 2C). No animal death and detectable Th2 cytokines (IL-4, IL-5, and IL-13) were observed after the challenge. The Th2 cytokine quantification was done by ELISA kits (R&D Systems, Minneapolis, MN).
3.3 The motifs inhibit lung DC maturation in response to HMPV
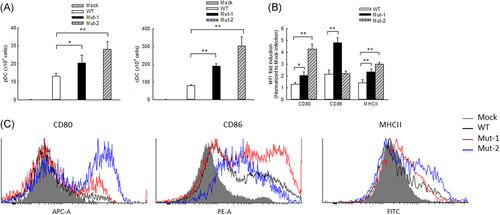
It has been previously shown that HMPV infection leads to the pulmonary recruitment of plasmacytoid DCs (pDCs) and conventional DCs (cDCs) with different kinetics after the primary infection of mice.44 Recently, we also demonstrated that monocyte-derived DCs (moDCs), an appropriate model for lung cDCs,45, 46 are regulated by the M2-2 protein in response to HMPV infection.20 Herein, we investigated whether M2-2 uses its putative PDZ-binding motifs to regulate the DCs in rHMPV-infected lungs. Fluorescence-activated cell sorting (FACS) analysis revealed that the number of lung pDCs and cDCs was increased after mutant inoculation compared with WT inoculation and this increase was most significant in Mut-2-infected mice on day 7 p.i. (Figure 3A). In addition to enhanced cDC infiltration, we also found the cDC maturation was affected by the motifs. In brief, lung cells isolated from WT- or mutant-infected mice were stained with DC maturation markers of CD80, CD86, or MHCII and followed by FACS analysis. At day 7 p.i., CD11c+ lung cells from Mut-1- and Mut-2-infected mice had a significant increase in the expression of CD80 and MHCII (Figure 3B,C) and Mut-1 had more impact on CD86 expression than WT-infected lungs. These results supported that M2-2 PDZ-binding motifs have an inhibitory effect on DC maturation and recruitment in HMPV infection.
3.4 The motifs suppress pulmonary T cell infiltration and activation in response to HMPV
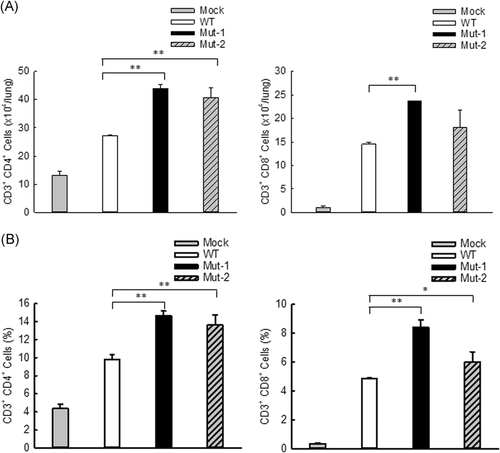
DCs play an essential role in bridging innate and adaptive immunity.47 DC maturation identified by costimulatory molecules including CD80 and CD86 is important for T cell function.48, 49 Improved DC maturation by mutant infection, therefore, likely leads to enhanced T cell responses. To test this hypothesis, pulmonary T cells were assessed at day 7 p.i. in mice infected with WT or mutants through FACS analysis. Compared with WT infection, the number of lung CD4 and CD8 cells was increased by Mut-1 infection (Figure 4A). Mut-2 infection also led to significant enhancement in CD4 cells, but not in CD8 cells. As shown in Figure 4B, the percentage of both CD4 and CD8 T cells was significantly enhanced by mutants, compared with pulmonary T cells of WT-infected lungs. Collectively, these results suggest that M2-2 PDZ-binding motifs are important in regulating T cell responses to HMPV infection.
4 DISCUSSION
As mentioned, the PDZ domains 29-DEMI-32 and 39-KEALSDGI-46 of M2-2 play an important role in host innate immune response in vitro18, 21, 22. However, the overall regulatory role in pulmonary innate responses to HMPV has not been investigated, although such studies are of significant importance to the development of therapeutic and preventive methods against HMPV infection.50 Herein, we demonstrated these two motifs are important in regulating HMPV replication and HMPV-induced pulmonary innate immunity. The attenuated motif mutants not only enhanced pulmonary innate immunity but protected immunized animals from naïve HMPV challenge without enhanced diseases. The enhanced innate immunity by motif mutants includes improved induction of many pulmonary immune mediators, better pulmonary recruitment of DCs and T cells, and more mature pulmonary DCs. Unlike heat-inactivated HMPV,6 Th2 cytokines including IL-4, IL-5, and IL-13, were not detectable following the primary mutant infection. Formalin-inactivated HMPV induces hypersensitivity in immunized animals following HMPV challenge.7 However, we did not observe enhanced Penh following HMPV challenge in mutant immunized mice (Figure 2C). All of these suggest that modification of M2-2 PDZ-binding motifs could be an approach to overcome poor mucosal innate immunity induction by HMPV. We also found that, following the primary infection, the effects of Mut-1 and Mut-2 on the induction of DC maturation markers, pulmonary function (Penh), and the production of cytokines/chemokines were distinct, suggesting the regulation of HMPV-induced innate immunity is motif-specific. In additional studies, we will investigate whether non-PDZ motifs, if there are any, also play a role in the immune evasion upon HMPV infection.
We also found that motifs differentially regulate DCs (Figure 3). In addition to the distinct induction of chemoattractants by two mutants, there are several other possibilities contributing to the motif-dependent regulation of DCs. Mutants may produce different pathogen-derived antigens, which may stimulate DCs differently. Motifs may control cellular signaling and cytoskeleton reorganization in DCs differently, leading to a distinct expression of their maturation markers in response to mutant infections.49, 51 AECs, our proteomics data have revealed that there are many other changes than motif-controlled immunity, such as pathways involved in cell signaling, chromatin remodeling, and oxidative responses.24 Overall, our finding demonstrates that M2-2 motif mutations are beneficial to induce a robust pulmonary innate immune response to HMPV infection.
In DCs, toll-like receptors (TLRs) and helicase melanoma differentiation-associated gene 5 (MDA5), two major pattern recognition receptors (PRRs), are essential for HMPV-induced innate immune responses.18, 20, 52-55 MDA5 uses MAVS as a central adaptor for antiviral signaling, while most TLRs, except TLR-3, use MyD88 to carry out antiviral responses.54, 56 We have recently shown that MAVS and MyD88 are critical to pulmonary immune responses to HMPV.25, 26 We also discovered that M2-2 hijacks MAVS and MyD88 to evade the immune response, and two M2-2 PDZ-binding motifs are responsible for such immune inhibition in AECs by suppressing the interaction between MAVS and its downstream effectors.18, 20, 21 Whether M2-2 also uses these two motifs to suppress MyD88-mediated immune responses in DCs remains to be investigated.
The study also demonstrated the importance of M2-2 PDZ-binding motifs in T cell responses. In the context of HMPV infection, T cells play essential roles in immune surveillance, protection, and viral clearance.27, 57 A defective CD8 T cell response delays HMPV clearance.58 In addition, memory CD8 T cells are capable of providing protection against a secondary infection.59 The induction of CD8 T cells by peptide immunization reduces viral load but induces lung pathogenesis upon the HMPV challenge.57 Although CD8 T cells have been suggested to cause pathogenesis upon HMPV challenge, mutant-enhanced T cell response seemed not to impact disease severity following challenge, based on Penh in WT- and mutant-infected mice (Figure 2C), suggesting that viral- and peptide-induced CD8 T cells may function differently from live virus-induced CD8 T cell response upon HMPV challenge.
A promising aspect of our mutants is their attenuated pulmonary replication. A recent study has identified the existence of defective interfering RNAs (DIs) in HMPV particles which are generated upon in vitro passage and able to induce type I IFN.60 Since HMPV replication is sensitive to the IFN, the attenuation of mutants might result from more DI production upon mutant infection. However, this is doubtful, as DIs are accumulated when virus stocks are prepared at multiple passages (of 5) with a high MOI (of 3). HMPV stocks used in this study were prepared at low passage (no more than 4-5 passages but passage 2 was mostly used) with a low MOI (approximately 0.01),21 suggesting that DIs are not likely to accumulate. Furthermore, Mut-2 did not produce more type I IFN than WT (Figure 1C). This result is somewhat different from what we found for the role of 39-KEALSDGI-46 in IFN-β induction in AECs. The difference between in vitro and in vivo settings may result from the involvement of other pulmonary cells in IFN-β induction and possible cell-type-dependent responses to Mut-2 infection. Our previous report also showed that mutant viruses are attenuated in Vero cells, which area cell line deficient of type I IFN signaling,21 which suggests that attenuation of mutant viral replication could be independent of DI production.
Several studies have demonstrated that the HMPV mutant with the deletion of M2-2 is a promising live-attenuated vaccine candidate.12, 61, 62 The M2-2 deletion mutant is highly attenuated and immunogenic in animals, however, the underlying mechanisms are not clear. Compared with the M2-2 deletion mutant, our motif mutants may have additional advantages. For example, our mutants have an intact H-2D-restricted cytotoxic T-lymphocyte (CTL) epitope which contributes to the clearance of HMPV-infected cells by T cells.57 In addition, the deletion of the whole M2-2 leads to a higher mutation frequency of HMPV.61 We found our motif mutants did not produce additional mutations at passages 2 or 3 (data not shown). In summary, our M2-2 PDZ-binding motif mutants were attenuated, elicited higher innate immune responses, and induced higher DC maturation and the recruitment of T cells and DCs in mouse lungs.
ACKNOWLEDGMENTS
Authors thank Cynthia Tribble for assistance with manuscript editing. This study was supported by grants from the National Institutes of Health-National Institute of Allergy and Infectious Diseases 1R01AI116812 and R21 AI113771, and the Clinical Innovator Award from the Flight Attendant Medical Research Institute to X.B.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
EC and XB designed the experiments, analyzed the data, and wrote the paper; WW prepared all rHMPV; YC, WY, LL, and AC performed the experiments.




