Construction of a recombinant-BCG containing the LMP2A and BZLF1 genes and its significance in the Epstein-Barr virus positive gastric carcinoma
Abstract
The signal peptide Ag85B of Bacillus Chalmette-Guerin (BCG) was used to construct a recombinant plasmid of BCG. The BCG-Ag85B gene and fused EBV LMP2A and BZLF1 genes were amplified and successively inserted into the Escherichia coli-BCG shuttle-vector pMV261. The recombinant plasmids were then amplified in E. coli DH5α and transformed into competent BCG. The expression of BZLF1 and LMP2A fusion proteins in recombinant-BCG (rBCG) was shown by Western blot. After the injection of recombinant-BCG into mice, antibodies against the fusion protein BZLF1 and LMP2A were measured by ELISA, and the cellular immune effects were determined by the lactate dehydrogenate (LDH) release assays. The results confirmed that the cloned genes of BCG-Ag85B and Z2A were correctly inserted into the vector pMV261. The recombinant plasmid pMVZ2A expressed Z2A in BCG effectively after transformation. The rBCG proteins were recognized by the BZLF1 (LMP2A) antibody. An ELISA demonstrated that rBCG could stimulate the generation of antibody against the fusion protein. The fusion gene was constructed successfully, and the rBCG induced humoral and cellular immune response in mice. J. Med. Virol. 86: 1780–1787, 2014. © 2014 Wiley Periodicals, Inc.
INTRODUCTION
Epstein-Barr virus (EBV) is a herpes virus with B-cell tropism. B-cells infected with EBV maintain latently the EBV genome as 172 kb circular plasmids. EBV is an oncogenic DNA virus that is the cause of a number of human malignancies including Burkitt's lymphoma, Hodgkin's lymphoma, gastric cancer, liver cancer, and nasopharyngeal carcinoma, as well as B-lymphocyte proliferative disease in immune-deficient patients. The International Agency for Research on Cancer of the World Health Organization classified EBV as a first category oncogenic factor in 1997 [Portis and Longneeker, 2003, 2004; Chen et al., 2005]. Therefore, the development of safe and effective EBV vaccines is a critically important global health priority. Since a host-vector system in mycobacterium was developed in 1987, recombinant-BCG (rBCG) technology has been applied extensively in the development of vaccines against a variety of infectious diseases, including bacterial, viral, and parasitic infections [Bastos et al., 2009]. BCG is an attractive vaccine vector because of its extensive safety record in humans, low production cost, heat stability, long-lasting induction of type 1 helper T-cell (Th1) immunity, activation of CD8 T-cells, adjuvant activity, pediatric use, mucosal immune induction, and oral administration. The serious epidemics of emerging and reemerging disease caused by EBV occur mainly in developing African and Asian countries. Consequently, BCG-based vaccines would be desirable globally due to low production costs and high heat stability [Matsuo and Yasutomi, 2011]. Furthermore, BCG also induces anti-tumor cytokines, which play an important role in cancer treatment [Gunven et al., 1978; Dhar et al., 2003; Sinn et al., 2008; Rosevear et al., 2009].
In this study, the fusion gene Z2A, which includes the immediate early gene BZLF1 and the LMP2A gene that encodes the EBV latent membrane protein, was constructed and inserted into the plasmid pMV261. After transfection into BCG, the Z2A fusion gene construct also expressed the secretory products of BCG, which could evoke an immune response in mice. This construct provides the basis for the development of Mycobacterium tuberculosis-EBV bivalent vaccines.
MATERIALS AND METHODS
Reagents
Taq DNA polymerase, T4 DNA ligase, 2 kb DNA marker, the restriction enzymes EcoRI, and SalI and BamHI were provided by Takara Bio, Japan. The target gene sequencing analysis was from Shanghai Sangon Biological Engineering Technology and Services Company, China. DNA transfection kits were purchased from the Invitrogen Company Carlsbad, CA. The substrate solutions of lactate dehydrogenase was purchased from Roche Corporation (Nanjing, Jiangsu, China). The LMP2A and BZLF1 antibody were from Abcam, USA. The ELISA kit was purchased from R.D., USA.
Bacterial Strains, Plasmids, Cultures, and Animals
BCG Danish strain (lyophilized powder, 50 g/l) was purchased from Shanghai Institute of Biological Products. pMV261 was a gift from Dr. Stover. Mycobacterium bovis BCG and rBCG were grown on Middlebrook 7H9 medium (Difco Laboratories, Detroit, MI) supplemented with 0.5% glycerol, 0.05% Tween 80, and 10% albumin-dextrose-catalase or on solid Middlebrook 7H11 medium (Difco Laboratories) supplemented with oleic acid-albumin-dextrose-catalase. When required, the antibiotic kanamycin was added at a concentration of 25 µg/ml. Escherichia coli DH5α was grown in Luria-Bertani medium and used for cloning. Healthy C57BL/6 male mice, 6–8 weeks of age (Beijing Vital River Com., Beijing, China) were bred under pathogen-free conditions and were fed autoclaved food with water available ad libitum.
Methods
The LMP2A and BZLF1 cDNAs were cloned from an EBV-positive cell line by RT-PCR and the fusion genes (LMP2A-linker-BZLF1) were constructed using a polypeptide linker (Gly4Ser)3 with spliced overlap extension (SOE). According to the open reading frame sequences of LMP2A and BZLF1, specific primers of these genes were designed with DNA star software. At the 5′ end of the upstream primer of LMP2A, an EcoRI restriction site and the protective bases GGC were included. The stop codon (TAA) was deleted from the reverse primer and the linker was included at the 3′ end. At the 5′ end of the primer for BZLF1, SalI restriction sites and the protective bases GC were included and the linker was introduced at the 3′ end of the reverse primer. The polypeptide linker (Gly4Ser)3 was composed of 15 amino acids encoding a hydrophobic polypeptide DNA sequence. The sequence was 5′-GGTGGCGGTGGAAGCGGCGGTGGCGGAAGCGGCGGTGGCGGCAGC-3′. The forward primer for the LMP2A F1 sequence was: 5′-GGCGAATTCATGGGGTCCCATGAAATGGTG-3′. The sequence of the overlapping downstream primer F2 was: 5′-GCTGCCGCCACCGCCGCTTCCGCCACCGCCGCTTCCACCGCCACCTACAGTGTTGCGATATGGGGT-3′. The BZLF1 overlapping upstream primer F3 sequence was: 5′-GGTGGCGGTGGAAGCGGCGGTGGCGGAAGCGGCGGTGGCGGCAGCATGATGGACCCAAACTCGAC-3′. The primer F4 sequence was: 5′-GCGTCGACATTAAGAGATCCTCGTG-3′. In the above sequences, the underlined portion indicates the linker, and the italicized portion indicates the restriction enzyme site. Primers were synthesized by Shanghai Sangon Biological Engineering Technology Services Limited synthesis. The amplified product sizes were 1,545 bp for LMPZAGCBF and 79 lbp for BZLFI. A 229 lbp Z2A fusion gene was subsequently created (both LMP2A and BZLFI included 45 pb of complementary linker; thus, the size of the fusion gene was 1,545 bp + 791 bp + 45 bp (Fig. 1)).
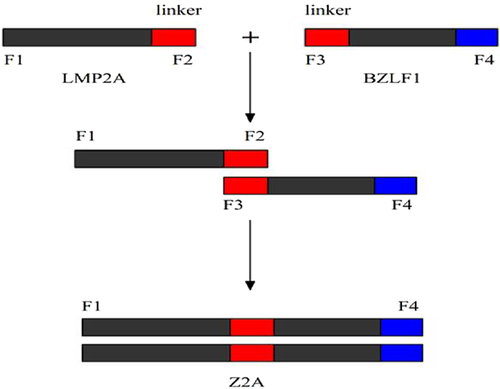
Primer Synthesis and PCR Amplification
- F: 5′-GATGGATCCAATGACAGACGTGAGCCGAAAG-3′;
- R: 5′-GTAGAATTCCGCGCCCGCGGTTGCCGCTCC-3.
A BamHI restriction site was introduced at the 5′ end of the F primer, and an EcoRI restriction site was added at the 3′ end of the R primer.
- F: 5′-AGCGAATTCATGGGGTCCCTAGAATGGTG-3;
- R: 5′-AGCGTCGACTTAGAAATTTAAGAGATCCTCGTG-3.
An EcoRI restriction site was introduced at the 5′ end of the F primer, while a SalI restriction site was introduced at the 5′ end of the R primer. The PCR reaction was performed in a single tube under following conditions: 94°C 5 min for pre-denaturizing; 30 cycles of 94°C 30 s for denaturizing, 54°C 50 s for annealing, 72°C 1.5 min for extension and then 72°C 8 min. PCR products were purified using a U-NIQ-5PCR product purification kit (Shanghai Sangon Products) according to the manufacturer's instructions. PCR products were detected by gel electrophoresis using a 1% agarose gel.
Construction of Recombinant Plasmid pMVS
The alkaline lysis method was used to extract and purify the plasmid pMV261 [Sambrook et al., 1989]. The signal peptide genes of BCG-Ag85B and plasmid pMV261 were digested using the restriction enzymes BamHI or EcoRI, respectively. Phosphorylation was carried out at 37°C for 1 h with 1 U calf intestinal alkaline phosphatase. The digested DNA fragments of the signal peptide gene and pMV261 were separated by electrophoresis, followed by phenol: chloroform (1:1) extraction and ethanol precipitation and were then washed with 70% ethanol. The purified DNA pellets were dissolved in TE. Each ligation reaction included 5 µl (equivalent) Ligation Solution I, 1 µl T4 DNA ligase, and water up to 10 µl, which was then incubated at 16°C for 30 min (or overnight at 4°C). Competent E. Coli DH5α cells were prepared using an adopted calcium chloride protocol. Plasmid transformation was performed by heat shock at 42°C. Single colonies were selected from a kanamycin-containing plate and then cultured overnight at 37°C [Jacobs et al., 1987].
Screening and Identification of the Positive Recombinant Plasmid PMVS
Selected colonies were inoculated in 2 ml LB liquid medium (containing kanamycin 50 mg/L) and then cultured at 37°C overnight. The plasmids were purified from the cultured colonies using the alkaline lysis method. The size of extracted plasmid was evaluated by initial observation and the linear plasmid size was tested by gel electrophoresis. Sequencing was performed for further identification of the plasmids.
The Fusion of Gene Z2A With Plasmid pMVS and the Identification of Plasmid pMVZ2A
The restriction enzymes EcoRI and SalI were used to digest Z2A and the plasmid PMVS, followed by ligation and subsequent transformation. Bacteria was cultured and plasmid extraction was performed as previously described. The plasmid pMVZ2A was confirmed by double enzyme digestion and partial gene sequencing.
Preparation of Competent BCG and Construction of the Recombinant-BCG
The BCG culture was grown in Middlebrook 7H9 medium and shaken at 250 r/min at 37°C. The BCG culture was then transferred to a centrifuge tube at an absorbance value of 0.6 (OD600) and incubated on ice for 2 hr. The culture was centrifuged at 4°C, 8,000 r/min (10 cm centrifugal radius) for 15 min, and the supernatant was then discarded. The BCG cell pellet was re-suspended in 10 times the initial pellet volume of 10% cold glycerol broth. The procedure was repeated five times, and the BCG pellet was re-suspended in 300 ml (50 times of the original volume). The competent BCG cells were kept at room temperature.
A volume of 15 µl of the pMVZ2A plasmid (approximately 2.3 µg) was transformed into 250 µl of competent BCG cells (approximately 5 × 106 competent BCG/ml). The parameters of electrophoresis were: 0.4 cm electroporation cuvette, voltage 2.5 KV, capacitance 25 µF, resistance 1,000 Ω, and reaction time 11.6 ms. The transformed competent BCG cells were cultured at 37°C for 10 h, and then, 100 µl of the culture was inoculated on a kanamycin-containing (50 mg/l) Middlebrook 7H10 solid medium centrifuge test tube slant and incubated at 37°C to allow for colony growth. After 3–4 weeks of selection, positive clones were identified by acid fast staining and were then cultured in liquid while shaking at 300 r/min at 37°C.
Western Blot
The recombinant-BCG was cultured in Middlebrook 7H9 broth media containing 50 µg/ml of kanamycin and was incubated at 37°C while shaking at 200 r/min. When the rBCG cells reached a concentration of 1.5 × 106 cell/ml, Isopropyl β-D-1-Thiogalactopyranoside was added to induce protein expression and the culture was shaken at 45°C. The cells were harvested after 24 hr, washed in PBS with 0.05% Tween 80 and then re-suspended in 1/15-volume of immunoprecipitation assay buffer. The culture lysates were analyzed by Western blot hybridization with BZLF1 and LMP2A antibodies and goat-anti-mouse IgG at 4°C (Sigma Chemical).
Antibody Detection
Animal experiments were performed in accordance with the guidelines of Chinese Council on Animal Care. Specific pathogen-free, 6-week-old female C57BL/6 mice (Beijing Vital River Com., Beijing, China) were bred in separate cages in a biosafety laboratory and fed commercial mouse chow and water ad libitum. Mice were randomly divided into four groups: two test groups and two control groups with 15 mice/group. Group 1 included animals injected with 100 µl of 5 × 108 recombinant-BCG cells/ml. Group 2 included animals injected with 100 µl of 5 × 107 recombinant-BCG cells/ml. The mice in Group 3 were injected with 100 µl of 5 × 108 BCG cells/ml, and the mice in Group 4 were injected with 100 µl sterile PBS. The animals were immunized once a week for 3 weeks. Blood was collected from the tail of each animal every 2 weeks for 14 weeks after the initial immunization. Serum was then extracted and the antibody concentration was measured by ELISA. The recombinant antigens LMP2A and BZLF1 were prepared at a concentration of 2 µg/ml and then added to a 96-well plate (0.5 µg/well). The reaction of the serially diluted mouse serum samples with the Goat anti-mouse IgG Antibody were then analyzed by ELISA according to the manufacturer's protocol.
CTL Response
Both recombinant-BCG and BCG were cultured in Middlebrook 7H9 medium in a shaker at 37°C. Once the optical density reached 0.6 (OD600), the cultures were transferred to a 45°C shaker for 12 hr. The medium was then removed and the cells were washed twice with PBS on a clean bench. The cells were diluted in PBS to a concentration of 5 × 108 cells/100 µl and injected subcutaneously into 6–8 weeks old mice. Mice receiving only BCG or PBS were used as controls. The mice were immunized again 3 or 5 weeks later. After 7 weeks, the mice were sacrificed and splenic lymphocytes were obtained. Lactate dehydrogenase (LDH) release assays were used to detect the CTL killing rate against EBV-positive gastric cancer cells. Cell killing rate = [(the average value A of experimental group − control group value A of natural to release)/(the average value A of maximum release control group − the control group of natural release value A)] × 100%.
Measurement of Animal Tumor Immunotherapy
EBV-positive gastric carcinoma cells were cultured to an integration degree of approximately 80%, at which point the tumor cells were digested with 0.02% EDTA and 0.25% Trypsin, centrifuged at 1,500g for 5 min, washed with DMEM medium and counted under a microscope. The cell concentration was adjusted to 1 × 107 cells/ml with DMEM culture medium and the number of live cells was assessed by Trypan blue staining. If the number of living cells was greater than 99% of all the cells, they were then used for animal experiments.
The tumor cells were inoculated subcutaneously in ten C57BL/6 female mice (2 × 105/mouse). Nine days after the inoculation, experimental animals with growing tumors (diameter of 3–5 mm) were divided into two groups. Five randomized experimental animals were included in each group. In the experimental group, each mouse was injected with 5 × 108 rBCG cells in 250 µl PBS. The control groups were comprised of mice injected with 250 µl PBS, 5 × 108 BCG cells in 250 µl PBS or 5 × 108 BCG + pMV261 cells in 250 µl PBS, respectively.
Ten days after the first immunization, the animals were injected again following the same dosing strategy. The mice were observed three to four times a week and state, coat color, diet, and weight were closely monitored. The mice were killed 70 days post-inoculation. The tumors were collected as completely as possible, and then, blood, and fat were removed.
The tumor length (X) and the short diameter (Y) were measured with a Vernier caliper to calculate the tumor volume V = (X × Y2)/2.
RESULTS
Identification of the Recombinant Plasmid PMVS
The size of PMVS was larger than that of pMV261 as measured by gel electrophoresis after digestion with BamHI and EcoRI. The digested products of PMVS were used as templates for BCG-Ag85B signal peptide sequence amplification and the band of 139 bp PCR product was confirmed by 1% agarose gel electrophoresis (Fig. 2). Preliminary results showed that the signal peptide sequence-containing recombinant plasmid (PMVS) was successfully constructed.
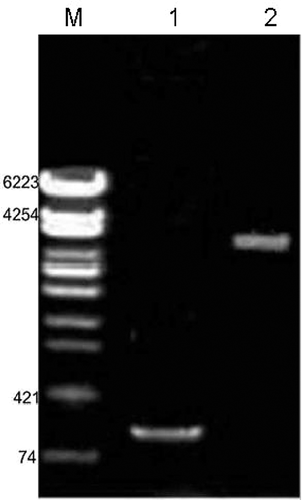
Identification of the Recombinant Plasmid pMVZ2A
To confirm the presence of EcoRI and SalI restriction endonuclease sites in pMVZ2A, Z2A was amplified by PCR assay with primers derived from both ends of the Z2A sequence. The insertion of the fusion gene fragment was detected by gel electrophoresis as a 2,291 bp band on a 1% agarose gel. The pMVZ2A plasmid was digested by BamHI and EcoRI, and a 139 bp signal peptide gene was observed by gel electrophoresis. The size of plasmid pMV261 + Z2A, which was consistent with calculated value, was approximately 6,900 (4,600 + 2,291) bp, as observed by gel electrophoresis (Fig. 3). The results showed that the recombinant plasmid pMVZ2A was constructed successfully.
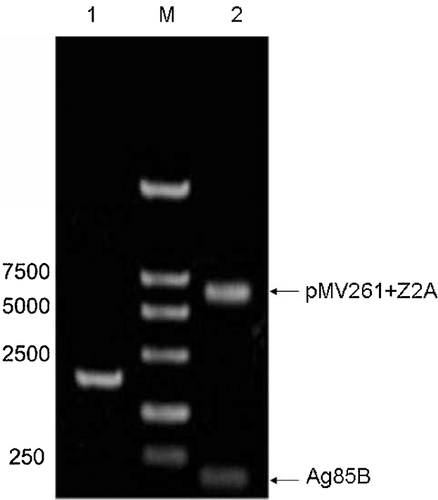
Further analysis of the positive recombinant plasmid pMVZ2A by sequencing revealed that the full-length of the insert was 139 + 2,291 bp, which was consistent with the reported cDNA sequence of the signal peptide and Z2A. From the pMV261 physical map and plasmid construction validation procedures, we confirmed that the 139 bp gene fragment was inserted between the BamHI and EcoRI restriction sites, and that the 2,291 bp gene fragment was inserted between EcoRI and SalI restriction sites. Sequencing results confirmed that both the signal peptide and Z2A genes were inserted in plasmid pMV261.
Detection of Gene Express by Western Blot
Western blotting showed the expression of BZLF1 and LMP2A proteins in rBCG, while expression was not detected in recombinant-BCG containing the empty vector (p BCG) (Fig. 4).
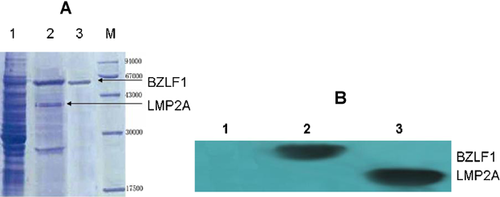
Humoral Immune Detection
Mice were immunized with 1 × 108 or 5 × 107 pfu recombinant-BCG and the level of antibody in the serum was detected by ELISA. At low doses, antibody production was relatively low, while the amount of antibody increased in response to the high dose of recombinant-BCG immunization (Fig. 5).
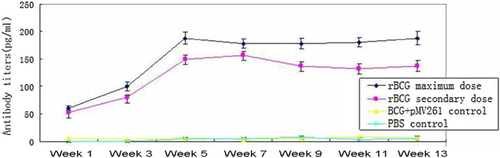
Specifically, before immunization, the antibody titer in the serum was close to 0. The week after the first immunization, the maximum dose of resulted in an antibody titer of 6500 (64.8 ± 7.4 pg/ml), while the lower dose resulted in a titer of 5300 (53.5 ± 4.2 pg/ml). The rBCG also stimulated antibody production more quickly. Six weeks after immunization, the maximum dose of resulted in an antibody titer of 18100 (180.5 ± 6.7 pg/ml), while the lower dose resulted in a titer of 16200 (162.3 ± 6.2 pg/ml). Seven weeks after immunization, the amount of antibody produced was steady in the rBCG groups, while the antibody titers from the control groups receiving either BCG or PBS remained close to 0 throughout the experiment. The difference was significant statistically (P ≤ 0.05).
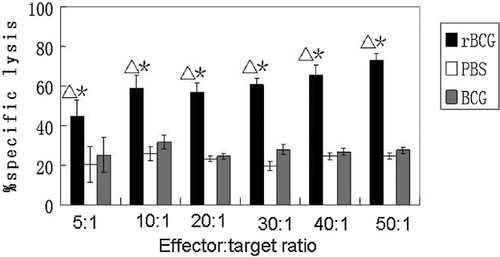
CTL Activation in Immunized Mice
CTL activation was measured in the recombinant-BCG experimental group, BCG control group and PBS control group to determine if recombinant-BCG could play a role in antigen-presentation and effectively stimulate a CTL response. The statistically significant results showed that the recombinant-BCG resulted in obvious CTL activation in mouse lymphocytes. Lactate dehydrogenated (LDH) release assays showed significant cytotoxicity of rBCG on EBV-positive tumor cells compared with BCG and PBS (P < 0.05). When the effector: target ratio was 50:1, the specific lysis was maximal and was approximately three times as strong as that in mice immunized with control BCG or PBS. There was no significant difference between the BCG and PBS groups (Fig. 6). These results show that rBCG was able to produce CTL cells, which then killed the EBV-positive gastric cancer cells in immunized animals.
The Effect of rBCG on EBV-Positive Tumor Growth in Mice
Compared with the control group, the tumorigenic ability was lower in the rBCG treatment group, with a tumor inhibition rate of 83.6%, indicating a statistically significant difference between the two groups (Table I). Furthermore, the tumor volume of the control group was significantly greater than that of the treatment group (Fig. 7).
| Groups | Numbers | Tumor size (mm3) | Inhibition rate (x ± s) |
|---|---|---|---|
| rBCG therapy group | 5 | 399.3 ± 108.8 mm3 | 0.90 ± 0.10 |
| BCG control group | 5 | 863.1 ± 129.8 mm3 | 0.07 ± 0.80 |
| pMV261 + BCG control group | 5 | 878.1 ± 139.3 mm3 | 0.10 ± 0.12 |
| PBS control group | 5 | 1,059.0 ± 409 mm3 | 0 ± 0.60 |

The Satterthwaite approximate t-test was used to confirm that the difference in tumor volume between the groups was statistically significant (t = 5.49, P = 0.0018, P < 0.01).
DISCUSSION
EBV is closely related with many tumors including large cell lymphomas, which are common in immunodeficient patients. Furthermore the treatment options for EBV-associated tumors are not effective.
With the development of tumor molecular biology, immunological methods have been used to prevent the occurrence of cancer and metastasis. These methods are beneficial preventative treatments and should draw attention from the medical community [Lutzky et al., 2010]. Vaccination is an important immunotherapy for the prevention and control of EBV infection.
The recombinant adenovirus vector expressing the EBV immediate early gene BZLF1 and the LMPA2 gene, which encodes the latent membrane protein gene, were constructed successfully, and the recombinant adenovirus vAd-Z2A was expressed efficiently in EBV-positive tumor cells. LMP2A induced specific CTL and the BZLF1 gene induced EBV from incubation phase to the lytic phase. Together, these genes had a synergistic effect on EBV-positive tumor cells. The fusion gene (Z2A) was expressed effectively in 293 cells with recombinant adenovirus [Yamada et al., 2002; Lou et al., 2006; Sun et al., 2006; Lin et al., 2011].
The fusion gene can also be expressed in EBV-positive tumor cells. The EBV gene LMP2A induced specific CTL and BZLF1 induced latent virus to lytic replication, which killed EBV-positive tumor cells synergistically. In this study, the BZLF1 (immediate early gene) and LMP2A (latent membrane protein gene) fusion gene (Z2A) of EBV was successfully constructed for further exploration. LMP2A contains restrictive MHC, virus-specific CTL recognition epitopes, which can be mediated by cytotoxic T-cells and play an important role in immune response [Barlow et al., 2009; Hutajulu et al., 2010; Hoebe et al., 2011]. For EBV-related tumors, the LMP2A protein is an ideal target antigen for immune therapy. Recent studies showed that LMP2A was expressed in 50% of EBV-associated gastric carcinomas. LMP2A was also consistently expressed in nasopharyngeal carcinoma, lymphoma and other tumor cells. LMP2A has T-cell activation potential epitopes, and the ability to activate CTL. Most importantly, LMP2A was not a transforming gene itself and non-carcinogenic [Wynne et al., 2010]. Redchenko and Rickinson [1999] used DC LMP2A epitope peptides to induce strong specific CTL immune responses.
In a healthy person, BCG can enhance the function of cellular and humoral immunity and stimulate T lymphocyte proliferation and excite delayed hypersensitivity. BCG also can enhance the immunogenic function of various antigens and accelerate immune responses, thus greatly enhancing the overall the immune response of tissues and organs [Ishii et al., 2005]. BCG sensitizes T lymphocytes to release cytokines and recruit macrophages at hypersensitivity reaction sites. The ribosomes, mitochondria, liposomes  and acid phosphatase of the activated macrophages increase significantly, resulting in increased phagocytic ability, which plays an important role in killing tumor cells. In addition, previous studies have demonstrated that the BCG can induce the production of IFN and TNF by CTLs, which is important for the killing or inhibition of tumor cells [Nadler et al., 2003; Wang et al., 2009]. It is therefore appropriate and reasonable to use BCG as the carrier.
and acid phosphatase of the activated macrophages increase significantly, resulting in increased phagocytic ability, which plays an important role in killing tumor cells. In addition, previous studies have demonstrated that the BCG can induce the production of IFN and TNF by CTLs, which is important for the killing or inhibition of tumor cells [Nadler et al., 2003; Wang et al., 2009]. It is therefore appropriate and reasonable to use BCG as the carrier.
BCG has been applied for nearly 100 years as a live attenuated vaccine. As a vaccine vector, BCG has the following advantages: (i) low toxicity, rarely causing complications; (ii) no effect on maternal antibodies; (iii) one of the most heat-resistant and widely used vaccines; (iv) ability to induce a sustainable immune response; (v) a strong adjuvant in experimental animals and human, which can effect macrophages and APCs and, thus, regulate the proportion of Th1-type and Th2-type cells [O'Donnell et al., 1994; Wangoo et al., 2000; Arnold et al., 2004; Yu et al., 2004; Liu et al., 2009].
In this study, a BCG shuttle-vector was successfully constructed and the rBCG could produce recombinant protein in vitro. Western blotting, ELISA and CTL activity were used to analyze the expression of the fusion gene. The experimental results showed that the rBCG vaccine could effectively inhibit the growth of tumors and induce a tumor-specific exclusion reaction, protecting the animal from EBV malignant transformation of tumor cells and inhibiting tumor cells in vivo. The experimental results also demonstrated that the rBCG vaccine was able to inhibit the growth of tumors effectively, resulting in the clearance of tumor cells in vivo, while BCG only modestly inhibited the growth of EBV-positive tumors. Therefore, the rBCG that was constructed successfully in this study has a greater anti-tumor effect than BCG.
ACKNOWLEDGMENTS
Shandon Nie, Yuanyuan Yang, and Wenjie Hu are acknowledged for their excellent technical assistance.




