Immunopathogenesis of dengue hemorrhagic fever: Contribution to the study of human liver lesions
Abstract
Dengue infection is an important tropical disease worldwide. The host immune response has been studied in order to better understand lesion mechanisms. It was performed an immunohistochemical study in 14 specimens of liver from patients with dengue hemorrhagic fever (DHF) to characterize cytokines and some factors present in liver lesions and their possible role in the pathogenesis of hepatic injury. Portal tract and hepatic acinus presented high expression of TLR2, TLR3, IL6, and granzyme B. Hepatic acinus also presented iNOS, IL18, and TGF-beta. Cells expressing IL12, IL13, JAk1, STAT1, and NF-κB were rarely visualized. Treg cells foxp3+ were absent. TLR2 and TLR3 seem to participate in cellular activation and cytokine production. Cytotoxic response seems to play a role. Although TGF-beta promotes the activation of Foxp3+ regulatory T cells, IL6 can significantly suppresses their generation. The expression of Treg cells is diminished probably as a result of the high frequency of these cytokines. Both cytokines play a role in the increased vascular permeability and edema observed in dengue liver specimens, with consequent plasma leakage and severity of the disease. It was observed a regular expression of IL-18 in hepatocytes and lymphocytes of the inflammatory infiltrate in portal tract, which reflects the acute inflammatory response that occurs in the liver and contributes to hepatic injury. At least in part, the increased number of cells expressing IL-18 could play a role of “up” regulation of FasL and correlate to the phenomenon of apoptosis, a mechanism of destruction of hepatocytes in DHF. J. Med. Virol. 86:1193–1197, 2014. © 2013 Wiley Periodicals, Inc.
INTRODUCTION
Dengue fever is a public health problem in tropical countries and is considered the most prevalent mosquito-borne viral disease worldwide. It is caused by a RNA virus that presents four genotypes, directly associated with the virulence and development of different clinical forms. The most severe form is dengue hemorrhagic fever (DHF)/dengue shock syndrome (DSS).
It has been speculated that the pathogenesis of dengue is multifactorial [Bhakdi and Kazatchkine, 1990; Bielefeldt-Ohmann, 1997; Rothman and Ennis, 1999]. Increased levels of some cytokines are detected in patients and seem to play a role in the establishment of DHF [Lin et al., 2005; Lee et al., 2007]. In this context, high levels of some cytokines were correlated with clinical presentation of DHF [Hober et al., 1993; Hung et al., 2004]. It was also demonstrated that endothelial activation induces an increased expression of those cytokines [Lin et al., 2005].
Viral tropism to the hepatic tissue, in which the dengue virus can replicate, has been described. Viral antigens are detected in hepatocytes and Kupffer cells [Couvelard et al., 1999]. Previously, it was performed an immunohistochemistry evaluation of the population of CD4 and CD8 T cells in hepatic lesions in DHF, which presented an increased number of both populations of cells in the portal area and zone 2 of acinar zone, with predominance of CD4 T cells. The expression of IFN-gamma predominated over IL4 and IL10 at that sites, characterizing a predominance of a Th1 profile of cytokines [Brasil, ].
The present study investigated the phenotype of these lesions with respect to the innate immune response (expression of Toll-like receptor 2 and 3) expression of proinflammatory cytokine (IL-6), adaptive (IL12 and IL18), Th2 (IL13), regulatory response (expression of Foxp3 and TGF-beta), participation of cytotoxic mediators (expression of granzyme) and signaling pathways (JAK1, Stat1, NF-κB). The aim is to contribute to the characterization of the immune response in hepatic lesions, focusing on the demonstration of some important cytokines and factors and their implication in the pathogenesis of DHF.
TLRs belong to innate immune response and are able to identify molecules from virus. The role of TLRs in viral infections by flaviviruses is little studied. It is known that TLR 3, 7, 8, and 9 are involved in yellow fever infection and TLR3 in West Nile virus, with an antiviral response. In dengue, experimental studies show that internalization of particles of DENV-2 induces the expression of TLR3 with subsequent release of proinflammatory cytokines.
IL6 plays a pivotal role in acute inflammatory reaction and is related to the generation of edema in inflammatory processes [Maruo et al., 1992; Akira et al., 1993]. In association with TGF-beta, IL6 inhibits the differentiation of Treg cells [Bettelli et al., 2006; Kimura and Kishimoto, 2010; Lee et al., 2007]. In DHF patients, this cytokine was related to severe disease [Marianneau et al., 1999]. TGF-beta is a functional factor produced by many cells and is responsible for multiple immunomodulatory effects. For example, this cytokine inhibits the proliferation of T and B cells and plays a role in apoptosis [Hsuan, 1989; Ding et al., 1990]. The regulatory T cells are described as a group of cells that participate in the mechanism of the immune system control [Belkaid and Rouse, 2005]. The most specific marker to this cell population is Foxp3 [Hori et al., 2003].
IL18 is secreted during some inflammatory disorders with consequent induction of other proinflammatory cytokines and adhesion molecules. IL18 can play different roles that seem to be dependable on the cytokine microenvironment. In association with IL4 naïve T cells develop into a Th2 patter. On the other hand, activated blood monocytes, hepatocytes, and Kupffer cells are able to produce IL18 and stimulate a Th1 response. The role of IL18 as a pro-apoptotic cytokine has been debated, although this cytokine is a potent inducer of NF-κB, which plays a role as an anti-apoptotic factor.
Janus kinase 1 (JAK1) is involved in the transduction pathways of IFN alpha, beta, and gamma. STAT is a protein activated by interferon-alpha, gamma, EGF, PDGF, and IL6. It is important in the expression of genes related to cell viability in response to pathogens.
MATERIALS AND METHODS
Retrospective cases of fourteen liver specimens from the archives of the Department of Pathology of Instituto Evandro Chagas, Pará, Brazil were obtained from viscerotomy of patients with DHF, collected from 1999 to 2001, according to the ethical standards of the institutional committee.
Diagnosis was confirmed by correlation of clinical data, by ELISA and histological examination by hematoxylin–eosin staining and detection of viral antigens by immunohistochemistry. The patients had presented clinical manifestation of hepatic injury, associated to systemic hemorrhagic manifestation such as positive tourniquet test, petechiae, and hemorrhagic skin suffusions.
The control group (n = 5) included liver specimens from patients who died without any infectious disease or liver damage, confirmed by histological evaluation.
Immunohistochemistry
Sections were obtained from formalin-fixed, paraffin-embedded liver samples, and re-hydrated by a series of increasing ethanol gradient. For antigen retrieval, sections were incubated in retrieval solution (Dako, S2367) pH9.0 for 20 min at 95°C. Immunohistochemical staining was performed by incubation with specific primary antibody (Table I). The tissue samples were incubated with the specific secondary antibody and a streptavidin–biotin peroxidase system, according to the manufacturer's instructions (LSAB system, code K0690, DakoCytomation, Carpinteria, CA). Each reaction was visualized with 3,3′-diaminobenzidine-tetrahydrochloride (DAB) (Sigma-Aldrich Chemical Co., St. Louis, MO). All slides were counterstained with Mayer's Hematoxylin and mounted for light microscope. Positive and negative controls followed each immunohistochemistry reaction. A semi-quantitative scale ranging from 0 for no staining, (+) for a discrete presence of positive cells, (++) for moderate, and (+++) for high number of positive cells was used to evaluate the results. Ten random fields were examined in portal tract, considering the positivity in mononuclear cells. Also, it was performed the semi-quantification in 20 random fields in hepatic acinus.
| Antibody | Portal area | Acinar zone | |
|---|---|---|---|
| Toll-like receptor 2 | SC8689/Santa Cruz, Biotech, Dallas, TX | +++ | +++ |
| Toll-like receptor 3 | Ab59919/ABCAM | +++ | +++ |
| iNOS | 482728/Calbiochem | + | +++ |
| IL6 | AF206NA/RD Systems | +++ | +++ |
| IL12 | MAB219/RD Systems | + | + |
| IL13 | AF213NA/RD Systems | + | + |
| IL18 | SC6178/Santa Cruz | + | +++ |
| TGF-beta | SC82/Santa Cruz | + | +++ |
| Foxp3 | 14477682/EBioscience | 0 | 0 |
| Granzyme B | SC1969/Santa Cruz | +++ | +++ |
| Jak1 | SC7228/Santa Cruz | + | + |
| Stat1 | 9176/Cell-Signaling | + | + |
| NF-κB | 3035/Cell-Signaling | + | + |
RESULTS
The histological alterations in the liver in DHF were localized frequently in the midzonal of acinar sites. Especially in the portal tract, it was observed an inflammatory infiltrate of lymphocytes, macrophages, and plasma cells, associated to edema.
The most prevalent and numerous markers in acinar zone were TLR2 and 3, iNOS, IL6, IL18, TGF-beta, and granzyme B. It was possible to observe the expression of IL6 in all lesions of DHF. The pattern of cytoplasmatic immunostaining was regular, diffuse and intense, with expression in mononuclear cells and lymphocytes, distributed in the inflammatory infiltrate in portal tract and hepatic acinus. All specimens presented cells expressing TGF-beta. Positivity was observed frequently in Kupffer cells and mononuclear cells, mainly in zone 2 of hepatic acinus. The expression of TGF-beta and IL6 in specimens from control group was practically absent.
A regular and diffuse expression of IL-18 was observed in 80% of cases of DHF, in Kupffer cells and hepatocytes in the form of particulate matter and also in lymphocytes of the inflammatory infiltrate in portal area. Cell expressing granzyme B was detected in all specimens primarily, the cytoplasm of mononuclear cells.
In a minor extent, some cells in acinar zone expressed IL12, IL13 and the factors JAk1, STAT1, and NF-κB.
Portal area was characterized primarily by high expression of TLR2, TLR3, IL6, and granzyme B. The remaining markers were rarely visualized (Fig. 1).
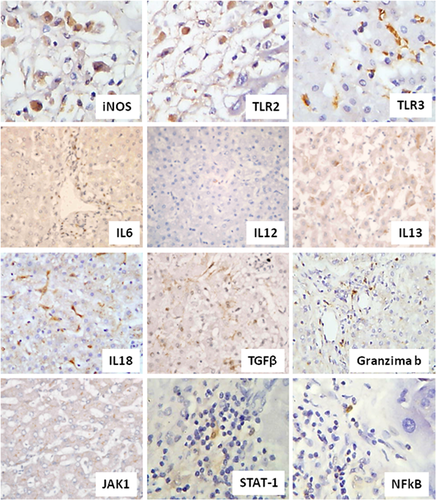
Cells expressing Foxp3 were absent in all the cases, both in portal tract or hepatic acinus. The results are summarized in Table I.
DISCUSSION
The pathogenesis of dengue infection has been explained as a result of the complex immune response, although the virulence of infecting dengue viruses is not excluded [Kurane, 2007]. In this context, understanding the pathogenesis must be based on T cell-mediated lesions, cross-reaction between endothelium and antibodies, C3, cytokine “Tsunami,” autoimmunity, and host genetic polymorphism.
Dengue is an acute inflammatory disease that affects the liver and determines important alterations mediated by apoptosis and necrosis of the hepatocytes [Huerre et al., 2001]. These alterations are associated to a scarce portal and intralobular inflammatory infiltrate of mononuclear cells. However there is intense edema and hemorrhagic phenomenon.
In this microenvironment the present data demonstrate an elevated number of cells expressing both IL6 and TGF-beta, but absence of cells expressing Foxp3. The expression of TGF-beta at the sites of lesions could be related to a scarce inflammatory response and hepatocyte apoptosis, characterized by the presence of councilman bodies. Although TGF-beta promotes the activation of Foxp3+ regulatory T cells [Bettelli et al., 2006] reported that IL6 can suppresses significantly the expression of Foxp3 and Treg cell generation. IL-6 and TGF-beta are important cytokines, which probably contribute to increased vascular permeability and plasma leakage and consequent greater disease severity. The increased expression of these cytokines would be probably responsible for inhibiting expression of Foxp3.
Some cytokines, such as TNF-α, IFN-γ, IL2, and IL6 are known as vasoactive factors [Maruo et al., 1992; Beynon et al., 1993]. It was demonstrated that in acute dengue infection, Treg cells are functional and able to suppress the production of vasoactive cytokines such as TNF-α and IL6 in response to viral antigens. Interestingly, it is speculated that in severe disease this mechanism might not be sufficient [Lühn et al., 2007]. An increased expression of IFN-gamma and TNF-alpha in DHF liver specimens has been reported [Brasil, ]. Complementing those results, besides this suppressive effect on Treg cells, TGF-beta, and IL6 also seem to play a role in the increased vascular permeability observed in dengue liver specimens, with consequent plasma leakage and severity of the disease.
TLR3 acts mainly by triggering the innate antiviral immune response and its role in DENV infection was demonstrated in vitro [Tsai et al., 2009]. Confirming those results, in this study it was observed high expression of both TLR2 and TLR3 in the portal area and acinar region, demonstrating in situ in liver injury their participation in the immune response in DHF.
It is known that IL12 is responsible for the activation of the Th1 pattern of immune response. More recently, IL18 has been regarded as a “super Th1” cytokine and the high expression in cases of DHF reflects the acute inflammatory response that occurs in the liver and corroborates the pattern of immune response directed towards the Th1 pole, with high expression of IFN-gamma, as detected previously in this series of cases [Brasil, ].
IL18 is associated to a protective role against Fas-mediated liver injury as a potent inducer of NF-κB, which functions as an anti-apoptotic factor. Interestingly, it was detected as a low expression of NF-κB and an important histological characterization of apoptosis in the lesions.
The immunological characterization of the environment at the site of lesions contributes to the understanding of hepatic injury determined by dengue virus and is represented by preferential destruction of hepatocytes by apoptosis (increase of TNF-alpha and TGF-beta) and necrosis, to a lesser degree. The findings of increased expression of IL-18 in hepatocytes in the context of tissue damage could indicate the need for protection thereof with anti-inflammatory or therapeutic monoclonal anti-IL-18.
Large number of cells expressing iNOS were detected in acinar zone, confirming the high levels of iNOS in Kupffer cells in vitro [Marianneau et al., 1999].
Cytotoxic T cells are able to mediate liver damage in dengue releasing perforin and granzyme or the interaction of Fas–Fas ligand [Gagnon et al., 1999]. High expression of granzyme B was detected both in portal area and acinar zone, demonstrating in liver lesions the presence of such mechanism. It has been suggested that significant cellular infiltrates in dengue may occur, including viral-specific T cells that induce apoptosis and liver damage. The mechanism of apoptosis will be studied in depth in these lesions.




