Influence of mutation of the HFE gene on the progression of chronic viral hepatitis B and C in Moroccan patients
Abstract
The implication of hemochromatosis (HFE) gene mutations in chronic viral hepatitis remains controversial. The aim of the present study was to measure the frequencies of the common HFE gene mutations in Moroccan subjects with chronic viral hepatitis B and C and to assess their influence on the progression of liver disease. H63D and C282Y mutations were screened by the polymerase chain reaction followed by restriction fragment length polymorphism analysis in 170 chronic hepatitis B patients, 168 chronic hepatitis C patients, and 200 healthy controls. A very small proportion of patients infected with hepatitis B virus (HBV) or hepatitis C virus (HCV; 1.8% and none, respectively) were heterozygous for the C282Y mutation, that is, rates not statistically different from those observed in healthy control (2%, P > 0.05). Similarly, the frequency of the H63D allele was not significantly different between HBV (13.8%) or HCV (14.3%) patients and controls (13.5%, P > 0.05). Multivariate analysis showed that carriers of the H63D mutation infected with HBV are at higher risk to progress towards an advanced liver disease when compared with patients infected with HBV with wild-type (OR = 2.45, 95% CI = 1.07–5.58). In contrast, no association between HFE mutated HCV-infected patients and an increased risk of disease progression was found (OR = 1.24, 95% CI = 0.61–2.50, P = 0.547). In conclusion, in Morocco the frequency of the HFE C282Y allele is very low and H63D mutation carriage occurs in almost 14% of the patients, a rate similar in chronic hepatitis patients and healthy controls. In the case of chronic hepatitis B, the carriage of the H63D variant represents a risk factor of evolution towards a more active disease. J. Med. Virol. 83:2096–2102, 2011. © 2011 Wiley Periodicals, Inc.
INTRODUCTION
Hereditary hemochromatosis is an autosomal recessive disorder, characterized by excessive dietary iron absorption and subsequent deposition in the parenchymal cells of the liver, pancreas, heart, joints, and pituitary gland. The gene is mapped to the short arm of chromosome 6 close to the HLA-A locus [Simon et al., 1987]. Hemochromatosis was basically considered as a monogenic disorder, a perception reinforced by HFE gene identification [Feder et al., 1996]. However, additional genes responsible for non-HFE-associated hemochromatosis were identified recently [Pietrangelo, 2005]. C282Y missense mutation in the HFE, a G to A transition at nucleotide 845 was found to be strongly related to the occurrence of hereditary hemochromatosis [Feder et al., 1996; Hanson et al., 2001]. A second missense mutation in the HFE gene, C to G change at position 187 causes a His to Asp mutation at codon 63 (H63D), is found in around 4% of patients with hereditary hemochromatosis, but its role in iron overload is still debated [Feder et al., 1996; Gochee et al., 2002]. The H63D mutation is more prevalent than the C282Y mutation with approximately one-fifth of the Europeans are H63D heterozygotes [Merryweather-Clarke et al., 1997]. Individuals who are compound heterozygous for C282Y and H63D occasionally have iron overload in the diagnostic range of hemochromatosis, although the penetrance of such genotype is low [Feder et al., 1996; De Gobbi et al., 2004]. Similarly, homozygosity for H63D has been associated with iron overload, ranging from asymptomatic subjects to patients with typical hemochromatosis. As with compound heterozygosity for C282Y/H63D, the penetrance is low and the phenotypic presentation of this genotype varied considerably [Aguilar-Martinez et al., 2001]. Several studies showed the role of iron in modulating the course of viral hepatitis. Authors observed that patients with higher levels of serum iron or ferritin were less likely to achieve spontaneous recovery after acute hepatitis B virus (HBV) infection [Blumberg et al., 1981; Bonkovsky et al., 1997; Bonkovsky, 2002]. In addition, elevations serum ferritin levels and serum iron saturation percentage were also noted in patients with chronic hepatitis C [Di Bisceglie et al., 1992; Arber et al., 1994; Bonkovsky, 2002; Lin et al., 2006]. Several studies have also indicated that, among patients with chronic hepatitis C, there is an increased prevalence of the C282Y mutation [Kazemi-Shirazi et al., 1999; Bonkovsky et al., 2002]. Patients with the C282Y mutation had more-advanced hepatic fibrosis and decompensated liver disease more frequently in the case of concomitant chronic hepatitis C than patients without this mutation [Hezode et al., 1999; Bonkovsky et al., 2002; Erhardt et al., 2003; Pacal et al., 2007; Won et al., 2009]. Recently, Ferrara et al. [2009] showed that serum ferritin is a predictor of treatment outcome in patients with chronic hepatitis C.
Outside Europe, the impact of such mutations on the progression of chronic viral hepatitis remains largely unknown. In Morocco, available data were limited regarding HFE gene mutations [Ezzikouri et al., 2008]. In addition, only few studies have focused on the relationship between HFE mutations and the persistence of HBV infection [Mah et al., 2005; Sendi et al., 2005; Ghaziani et al., 2007]. The aim of the current study was to determine whether there is any difference between the frequencies of HFE mutations in patients with HBsAg and patients infected with hepatitis C virus (HCV), compared with an appropriate control group.
MATERIALS AND METHODS
Study Population
A total of 538 Moroccan patients were enrolled in the case–control study. Blood samples from chronic hepatitis B and C patients were collected from the Pasteur Institute of Morocco, Casablanca and Hospital University Center Ibn-Sina, Service of Medicine C, Rabat during a 6-year period (June 2004–June 2010). The case group comprised two subsets of patients: 170 patients (60 women, 110 men) with chronic hepatitis B (70 patients without symptoms and 53 with advanced liver disease) and 168 patients (82 women, 86 men) with chronic hepatitis C (107 HCV-related chronic persistent hepatitis and 68 HCV-related advanced liver disease. Diagnosis of chronic hepatitis B or C was based on the following criteria: (i) presence of the HBsAg or (ii) HCV antibody. Chronic HBV infection was defined as positive for HBsAg and chronic HCV infection as positive for anti-HCV and HCV ribonucleic acid (HCV-RNA) positivity over a period of more than 6 months, respectively. The controls consisted of 200 healthy (93 women, 107 men) unrelated subjects negative for viral hepatitis markers and normal serum levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST) and with no history of liver disease and anemia recruited from the Pasteur Institute of Morocco during the same time period. The controls were matched to the cases for sex and ethnicity. After providing informed consent, every participant was interviewed according to a questionnaire exploring demographic features as well as potential risk factors. These investigations were approved by the Ethics Comittee of the Faculté de Medicine of Casablanca.
Serological markers for hepatitis viruses were tested with commercial available kits (Axsym, Abbott Diagnostics, Wiesbaden-Delkenheim, Germany) for HBsAg, Hepatitis B e antigen (HBeAg), anti-HBe, anti-HBc IgG, anti-HBsAg, and anti-HCV antibody for cases and HBsAg and anti-HCV antibody for control subjects.
Molecular Analysis of HFE Mutations and Serum Ferritin Determination
Genomic DNA was isolated from peripheral leukocytes. Peripheral blood leukocytes were digested in SDS/proteinase K buffer at 37°C for 6–12 hr, followed by 2 phenol and 1 chloroform extractions. DNA was precipitated with ethanol and resuspended in TE buffer. The presence of missense mutations in the HFE gene (C282Y and H63D) was verified by means of restriction fragment length polymorphism of PCR products as previously described [Feder et al., 1996; Ezzikouri et al., 2008]. Briefly, a step-down amplification was performed at annealing temperatures of 50 and 52°C for analysis of H63D and C282Y mutations, respectively. The amplified fragments were digested with RsaI for the C282Y and MboI for the H63D mutations (Fermentas-Euromedex, Souffelweyersheim, France) for 16 hr at 37°C. Upon digestion with RsaI, the 387 bp PCR product of C282Y region shows two fragments of 247 and 140 bp in normal DNA and two additional fragments of 111 and 29 bp in mutant DNA. The 208 bp H63D region PCR product digested with MboI and digestion products were analyzed following electrophoresis in 3% agarose gel stained with ethidium bromide generates fragments of 138 bp, 70 bp in normal DNA, and 208 bp in mutant DNA. PCR digests were analyzed on 3% agarose gel. Repeated genotyping was routinely carried out for 10% of the samples for quality control purposes for all genotyping assays, with 100% concordance of results. Serum ferritin was determined with the Axsym analyser (Axsym, Abbott Diagnostics, Wiesbaden-Delkenheim, Germany).
Statistical Analysis
One-way analysis of variance was conducted to compare two means. Fisher's exact test and Mantel–Haenszel were used where appropriate. Departures from Hardy–Weinberg equilibrium (HWE) were determined by comparing the observed genotype frequencies with expected genotype frequencies calculated using observed allele frequencies by Pearson's goodness-of-fit chi-square. The odds ratio (OR) and 95% confidence interval (CI) were calculated using an unconditional logistic regression model and adjusted by age and gender. A P-value of <0.05 was considered statistically significant. All tests were two-sided.
RESULTS
A total of 538 participants were enrolled in the survey (Figs. 1 and 2). Cooperation rates (i.e., the number of individuals who were interviewed divided by the number interviewed plus the number of individuals who refused to be interviewed) were 87% for case patients and 70% for control subjects. A case–control study investigating the prevalence of HFE mutations in patients either with chronic HBV carriage (n = 170), or infected with HCV (n = 168) and 200 control individuals was conducted (Figs. 1 and 2). Table I shows the characteristics of the groups studied.
Analysis of H63D mutation of PCR-RFLP: M, 100 bp ladder. His/His: histidine acid, wild-type genotype or H/H. Asp/Asp: aspartic acid, mutated genotype or D/D.
Analysis of H63D mutation of PCR-RFLP: M, 100 bp ladder. C/C: Cysteine acid, wild-type genotype. CY: Tyrosine acid, mutated genotype.
| Healthy controls, N = 200 | Hepatitis C patients, N = 168 | P-value* | Hepatitis B patients, N = 170 | P-value† | |
|---|---|---|---|---|---|
| Mean age ± SD (years) | 54.17 ± 10.36 | 63.07 ± 9.96 | <0.0001 | 42.41 ± 11.44 | <0.0001 |
| Gender (%) | |||||
| Male | 53.50 | 51.20 | 0.676 | 64.70 | 0.121 |
| Female | 46.50 | 48.80 | 35.30 | ||
| Aminotranferases | |||||
| ALTa | 30.16 ± 1.13 | 70.85 ± 5.40 | <0.0001 | 56 ± 3.80 | <0.0001 |
| ASTb | 26.41 ±1.60 | 70.12 ± 4.95 | <0.0001 | 46.41± 4.18 | <0.0001 |
| Ferritin (ng/ml) | 80.84 | 146.67 | 0.229 | 110.7 | 0.607 |
| Viral load (IU/ml)c | ND | 439,500 [1,310–4,550,000] | 1,800 [15–109] | — | |
- a ND, not determined.
- b Normal value [7–56].
- c Normal value [5–35].
- d Median.
- e HCV infected versus healthy controls.
- f HBV infected versus healthy controls.
Genotype and allele frequencies of the C282Y and H63D substitutions among case patients and control subjects are provided on Table II. Testing for departure from HWE revealed that all groups respects the HWE (P > 0.05) but is not for the C282Y polymorphism in hepatitis C group (P = 10−5). A total of 200 subject controls were studied: 4 (2%) were found to be heterozygous for the C282Y mutation and 48 (24%) heterozygous and 3 homozygous (1.5%) for the H63D mutation. Allele frequencies were 13.5% for H63D and 1% for C282Y (Table II).
| Genotype frequencies, N (%) | Allele frequencies | |||||
|---|---|---|---|---|---|---|
| CC | CY | YY | C (%) | Y (%) | P-value | |
| HFE C282Y polymorphism | ||||||
| HCV patients (N = 168)a | 168 (100) | 0 (0.0) | 0 (0.0) | 100 | 0 | NS |
| HBV patients (N = 170) | 166 (97.6) | 4 (2.4) | 0 (0.0) | 98.8 | 1.2 | NS |
| Controls (N = 200) | 196 (98) | 4 (2.0) | 0 (0.0) | 99 | 1 | |
| HH | HD | DD | H (%) | D (%) | P-value | |
|---|---|---|---|---|---|---|
| HFE H63D polymorphism | ||||||
| HCV patients (N = 168) | 123 (73.2) | 42 (25.0) | 3 (1.8) | 85.7 | 14.3 | NS |
| HBV patients (N = 170) | 127 (74.7) | 39 (22.9) | 4 (2.3) | 86.2 | 13.8 | NS |
| Controls (N = 200) | 149 (74.5) | 48 (24.0) | 3 (1.5) | 86.5 | 13.5 | |
- HBV, hepatitis B virus; HCV, hepatitis C virus; NS, not significant.
- a Departure from Hardy–Weinberg equilibrium (P < 0.05, χ2 test).
Homozygosity YY at codon 282 was absent from the subjects studied. Among the 170 HBsAg positive patients, 4 HBV patients (2.4%) were heterozygous for the C282Y mutation, 39 (22.9%) patients were H63D heterozygous, 4 (2.3%) patient were homozygous for H63D (Table II). Of the 168 subjects with chronic hepatitis C, none were heterozygous for the C282Y mutation, 42 (25%) for the H63D, and 3 (1.8%) were homozygous for H63D (Table II). Proportions of HFE mutations were not significantly different between patients with chronic hepatitis B or C and the controls (27% or 26.8% vs. 26.5%, 0.812 < P < 0.964).
Comparisons of genotype frequencies were extended to categorical or continuous variables. The prevalence of HFE mutations was not significantly different between patients with chronic hepatitis B or C with and without mutations according to gender, viral loads, or transaminase levels (Fig. 3). Adjusting for age and sex, serum ferritin levels were not significantly different between HBV and HCV patients and controls with HFE mutations (110.7 ± 43.61 ng/ml vs. 80.84 ± 21.38 ng/ml, P = 0.607, 149.67 ± 43.52 ng/ml vs. 80.84 ± 21.38 ng/ml, P = 0.229, respectively).
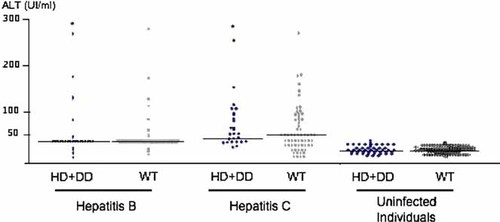
Dot plot representing the levels of alanine aminotranferase (ALT) in IU/ml. Patients and controls were stratified according to their infection status and HFE H63D genotype. Horizontal bars represent the median value in each group. No significant difference was observed between carriers of H63D mutation and wild-type individuals within each class (hepatitis B, hepatitis C, uninfected control individuals).
To test the association between the presence of the C282Y/H63D and progression of the liver diseases, the prevalence of mutations were examined according to the histological status in the liver of patients. A subset of 53 patients with advanced liver disease and infected with HBV (patients with chronic active hepatitis, cirrhosis, and hepatocellular carcinoma) was compared with the remaining 70 asymptomatic HBV carriers (Table III). The frequency of C282Y was 0.9% in patients with advanced liver disease and 0.14% in asymptomatic HBV carriers. However, this difference was not significant (P = 1.000). By contrast, the frequency of the aspartate variant at codon 63 was 21.7% in advanced liver disease and only 9.3% in asymptomatic carriers, that is, a difference statistically significant (P = 0.006). The frequencies of the homozygous D/D genotype at codon 63 were 7.5% and none in patients with advanced liver disease and asymptomatic HBV carriers, respectively (P = 0.012). The prevalence of homozygous (DD) and heterozygous (HD) carriers was also significantly higher in patients with advanced disease when compared with asymptomatic carriers (35.8% vs. 18.57% respectively, P = 0.030). Multivariate logistic regression analysis for HBV-advanced liver disease and H63D mutation compared with H63D wild-type after adjustment for age and sex revealed an OR of 15 (95% CI: 0.78–287.18, P = 0.012; Table III).
| HFE mutation | Asymptomatic HBV carriers (n = 70)a | HBV-advanced liver disease (n = 53)b | OR (95% CI)c | P-value |
|---|---|---|---|---|
| H63D | ||||
| H/H | 57 | 34 | 1.00 | |
| H/D | 13 | 15 | 1.93 (0.82–4.55) | 0.127 |
| D/D | 0 | 4 | 15 (0.78–287.18) | 0.012 |
| H/D + D/D | 13 | 19 | 2.45 (1.07–5.58) | 0.030 |
| Allele | ||||
| H | 0.907 ± 0.023 | 0.783 ± 0.043 | 1.00 | |
| D | 0.093 ± 0.023 | 0.217 ± 0.043 | 2.70 (1.30–5.64) | 0.006 |
| C282Y | ||||
| C/C | 68 | 52 | 1.00 | |
| C/Y | 2 | 1 | 0.65 (0.06–7.41) | 0.730 |
| Y/Y | 0 | 0 | 1.30 (0.02–66.85) | 1.000 |
| Allele | ||||
| C | 0.986 ± 0.010 | 0.991 ± 0.009 | 1.00 | |
| Y | 0.014 ± 0.010 | 0.009 ± 0.009 | 0.66 (0.06–7.34) | 1.000 |
- a Including patients with normal transaminases at least three times during the years of enrollment and viral load <2,000 IU/ml.
- b Including patient with active chronic carriers, liver cirrhosis, and hepatocellular carcinoma.
- c Adjusted for age and gender.
Next, we examine the impact of HFE on the progression of HCV-associated disease infection (Table IV). In contrast with the situation observed with HBV, a similar analysis for HCV-related advanced liver disease and H63D mutation did not reveal any significant association (OR = 1.24, 95% CI: 0.61–2.50, P = 0.547).
| HFE mutation | HCV-related chronic persistent hepatitis (n = 107) | HCV-related advanced liver disease (n = 68)a | OR (95% CI)b | P-value |
|---|---|---|---|---|
| H63D | ||||
| H/H | 80 | 43 | 1.00 | |
| H/D | 25 | 17 | 1.26 (0.62–2.59) | 0.521 |
| D/D | 2 | 1 | 0.930 (0.08–10.55) | 0.953 |
| H/D + D/D | 27 | 18 | 1.24 (0.61–2.50) | 0.547 |
| C282Y | ||||
| C/C | 107 | 68 | ||
| C/Y | 0 | 0 | — | — |
| Y/Y | 0 | 0 | — | — |
- a Including patient with liver cirrhosis and hepatocellular carcinoma.
- b Adjusted for age and gender.
DISCUSSION
The inherited mutations in the HFE gene such as C282Y and H63D are a common cause of iron overload and may accelerate the process of liver fibrosis in patients infected with hepatitis B and C viruses [Drakesmith and Prentice, 2008]. The prevalence of HFE gene mutation is known to vary significantly between the different ethnicities. Until now, most of the studies on the iron overload in chronic hepatitis B and C were performed in Western countries and in Asian-Pacific area, whereas in Africa the data are very limited. The present study was undertaken to estimate the prevalence of the mutations in HFE in a large series of Moroccan patients with hepatitis B and C. Blumberg et al. [1981] and Lustbader et al. [1983] were among the first to propose a relationship between body iron, outcome of hepatitis B, and clearance of HBV. They found that hemodialysis patients with higher levels of serum ferritin were more likely to develop persistent chronic hepatitis B after acute HBV infection. In support of their results, a beneficial effect in response to interferon therapy was achieved by reduction of serum ferritin levels in patients with chronic hepatitis B [Bayraktar et al., 1996, 1998]. The interaction between iron overload and hepatitis B was substantiated further by a prospective study in which patients with hereditary hemochromatosis and concomitant HBV infection were at increased risk of developing cirrhosis and HCC [Fargion et al., 1994]. The main result of the present large case–control study was that in a Moroccan population the prevalence of C282Y and H63D mutations did not differ between patients with chronic hepatitis B and healthy controls. In addition, the homozygous C282Y status was absent from individuals with chronic hepatitis as well as controls. Overall, these findings are in agreement with well documented studies from the Middle-East and eastern-Asia [Mah et al., 2005; Ghaziani et al., 2007] but differ from the report of Sendi et al. [2005] showing that in Iranians patients, the frequency of major mutation C282Y in HFE gene with HBV infection is higher than control subjects (4% vs. 0%, P = 0.02). However, it must be stressed that the latter study was performed on a limited number of patients (n = 75). In addition, the present report suggests that a deleterious interaction might occur between the H36D mutation and HBV infection. Patients infected with HBV and carrying this latter mutation are at higher risk of developing advanced liver disease (including cirrhosis and HCC) than wild-type individuals. Thus, these results keep in line with previous report [Fargion et al., 2001].
HCV infection is persistent in the majority of infected individuals, and carriers develop chronic liver disease with approximately 20% developing cirrhosis after 20 years [Lauer and Walker, 2001]. The natural history of chronic liver disease caused by HCV remains controversial with varying rates of progression to cirrhosis [Sklan et al., 2009]. The role of iron in chronic hepatitis C was examined recently. It has long been recognized that iron overload promotes hepatic fibrosis in hereditary hemochromatosis (reviewed by Drakesmith and Prentice, 2008). Serum iron stores are increased frequently in patients with chronic hepatitis C and elevated hepatic iron concentration has been associated with a poor response to interferon alfa [Ferrara et al., 2009]. In the present study, HFE gene mutations were assessed retrospectively in a large cohort of well-characterized chronic hepatitis C patients. We did not find any difference concerning the distribution of HFE genotypes in HCV patients and healthy controls group and there is no influence on HCV progression. This finding seems to be in agreement with the usual settings of HCV infection in Western and Central Europe [Thorburn et al., 2002; Erhardt et al., 2003; Pacal et al., 2007]. More specifically, the data presented confines the report of Thorburn et al. [2002] showing that carriage of HFE mutation was not associated with any clinical, biochemical, virological, or pathological features of intrahepatic iron overload during the chronic phase of HCV infection. Interestingly, Erhardt et al. [2003] also found that allele frequencies of the C282Y and H63D mutation did not differ between HCV patients and healthy controls (6.95% vs. 6.2%; 14.75% vs. 16.4%, respectively). By contrast, several studies of British or American patients showed that carriage of HFE mutations was associated with a deterioration of chronic hepatitis C and a poor response to antiviral treatment [Smith et al., 1998; Bonkovsky et al., 2002]. Smith et al. [1998] proposed an association between carriages of HFE mutations and increased hepatic fibrosis scores. In that study, 10 C282Y heterozygotes of whom 4 (40%) had cirrhosis were compared with 127 normal controls of whom 11 (8.7%) were cirrhotic (P = 0.01). Heterozygotes had more advanced liver disease (P = 0.01) and more commonly had iron staining on Perl's stained liver sections (P = 0.02). Another study by a different group supported this assumption [Pirisi et al., 2000]. In a previous study it was found that H63D carrier state is significantly associated with an increased risk of hepatocellular carcinoma in Moroccan men (P = 0.001) [Ezzikouri et al., 2008]. Interestingly, Bonkovsky et al. [2002] showed that HFE mutations are associated with increased iron loading in advanced chronic hepatitis C patients; however, the presence of HFE mutations, in particular the H63D mutations, was associated with an increase of sustained viral response. The above-mentioned discrepancies might result (apart from other factors such as different study designs and statistical powers) from the variation of prevalence of HFE mutations among populations, at least in Europe, where a decreasing north to south gradient is well described. The prevalence of C282Y and H63D HFE mutations in the Moroccan population is generally lower than that reported in patients infected with HCV from North and Western Europe.
In patients infected with HBV, adjusting for age and sex the mean level (±SD) of serum ferritin in subjects with the C282Y and H63D mutations (110.7 ± 43.61 ng/ml), although numerically higher, was not statistically different from that of subjects with HFE mutation in control subjects (80.84 ± 21.38 ng/ml, P = 0.607; reference ranges for serum ferritin: 20–290 ng/ml in men and 8–130 ng/ml in women). In chronic hepatitis C patients the mean level of serum ferritin in subjects with the C282Y and H63D mutations (149.67 ± 43.52 ng/ml) was not statistically different from those in patients with HFE mutation in control subjects (80.84 ± 21.38 ng/ml; P = 0.229). Overall, these results were compatible to studies by Thorburn et al. [2002] and Sendi et al. [2005]. However, several reports showed previously a significant difference in serum ferritin between patients with chronic Hepatitis B and C and healthy controls [Lebray et al., 2004; Ghaziani et al., 2007]. Patients with elevated serum iron markers were more commonly male, drank more alcohol, and had more active chronic hepatitis with increased liver fibrosis. This observation suggests that elevated serum iron markers in patients with chronic hepatitis viruses infection are the result of active hepatitis, either through leakage from damaged hepatocytes or as part of the systemic inflammatory response.
In Morocco, the frequency of the C282Y is rare and H63D carriage occurs approximately 13% of the subjects with or without hepatitis B or C. Taken together, the current study indicates, as previous studies performed in Europe but in contrast with British or American reports that HFE mutation does not predispose for chronicity of hepatitis B or C but predispose to progression of HBV-associated disease.
Acknowledgements
The authors would like to acknowledge all patients for their participation in this study.




