A rapid and efficient method BK polyomavirus genotyping by high-resolution melting analysis
Abstract
A small percentage of renal patients become infected with the BK polyomavirus (BKV), a pathogenic virus that causes BKV-associated nephropathy (BKVN), after kidney transplantation. This study presents a simple, rapid, high-throughput method for BKV genotyping using high-resolution melt analysis (HRMA). Using this novel method, BKV genotypes were analyzed in 49 samples taken from BKV-positive renal transplantation patients for classification into 1 of 3 genotypes: GI-1 (subgroups Ia, Ib1, and Ic), GI-2 (subgroup Ib2), and GII-IV (subtypes II, III, and IV). HRMA was performed to compare each sample sequence to a reference sequence that contained a combination of 2 of the 3 genotype groups, and the findings validated by conventional DNA sequencing. Of the 49 samples, 20 samples were classified as GI-1, 18 as GI-2, and 11 as GII-IV, suggesting that the predominant BKV strain (77.6%) in these patients was subtype I (GI-1 and GI-2). The HRMA method presented here is a time-saving, reliable, and low-cost procedure that can be developed as a diagnostic tool in the detection of the specific BKV genotypes associated with BKVN. J. Med. Virol. 83:2128–2134, 2011. © 2011 Wiley Periodicals, Inc.
INTRODUCTION
Post-transplant infection by reactivation and replication of the BK polyomavirus (BKV; NC_001538.1) can present as BKV-associated nephropathy (BKVN). This syndrome, which affects 1–5% of renal transplantation patients [Bonvoisin et al., 2008], is a major post-transplant complication characterized by persistent graft dysfunction and graft loss. The current recommendation for preventing BKVN or monitoring its development post-infection is assessment of BKV load in plasma and urine [Hirsch et al., 2005]. One reliable method of measuring BKV load is polymerase chain reaction (PCR) analysis of the urine and plasma of renal transplant patients at risk of developing BKVN. Although the viral load cutoff levels associated with nephropathy have not been definitively established [Tremolada et al., 2010b], a plasma viral load level >1 × 104 copies/ml and a urine viral load level greater than 1 × 107 copies/ml are associated with a higher risk of BKVN [Bonvoisin et al., 2008; Cimbaluk et al., 2009; Boldorini et al., 2009a]. An increase in plasma BKV load to greater than 1 × 104 copies/ml is associated with increased probability of a positive histological result [sensitivity, >95%; specificity, >95%; Randhawa et al., 2004]. The identification of these associations has led to the current recommendation that BKV load in the plasma and urine of renal transplant patients be monitored routinely after transplantation.
One notable feature of BKV is the remarkable diversity of its genomic sequences. The National Center for Biotechnology Information (NCBI) database contains data on more than 250 BKV whole-genome sequences that have been identified and classified by phylogenetic analysis [Luo et al., 2009]. It is this genomic diversity that is responsible for discrepancies in the research on the clinical relevance of BKV and its associations with BKVN. For example, whereas Krautkramer et al. [2009] found no correlation between mutations in BKV VP1 genes and BKV viral load, Boldorini et al. [2009b] identified a correlation between BKVN and the frequency of single base-pair mutations in BKV DNA. On the other hand, Chan et al. [2009] described observing a case of BKVN in which a BKV large-T antigen was unreactive. Adding to the uncertainty regarding the association between the BKV genotype and BKVN is the frequent detection of BKV polymorphism in the VP1 region of the virus in the urine of patients with BKVN [Tremolada et al., 2010a].
DNA sequencing, currently the most reliable method for studying DNA diversity, has become even more reliable with recent technological advances improving the melting analysis of double-stranded DNA, especially in the detection of DNA sequence variations. High-resolution melt curve analysis (HRMA), a key technology for the detection of single nucleotide polymorphisms (SNPs), is rapidly becoming the tool of choice for screening pathogenic DNA variants because of its ease of use, simplicity, flexibility, low cost, non-destructive nature, superb sensitivity, and high specificity with minimal post-PCR handling [Vossen et al., 2009]. Recognition of these benefits has led to much research into procedures for the rapid identification of DNA variants by HRMA [Lin et al., 2008; Steer et al., 2009]. However, as indicated by the incorrect identification of several DNA sequence variants with only 1 SNP by HRMA [Hung et al., 2009], HRMA must be optimized for targeting SNP in order to become a truly reliable and accurate method for screening pathogenic DNA variants.
Two particular research challenges to such optimization are the remarkable diversity of the BKV genomic sequence and the possibility that a particular SNP is unrelated to a specific genotype, both of which increase the difficulty of identifying a specific BKV genotype. To address these challenges, Iwaki et al. [2010] attempted to develop a quantitative PCR assay for a stable genomic region of the BK virus [Iwaki et al., 2010]. Using the same site in its BKV genotype analysis, the present study aimed to develop a more rapid and reliable yet technically less complex method of genotyping BKV using HRMA.
MATERIALS AND METHODS
BKV Subtype Analysis Based on Phylogenetic Analysis
In accordance with the method described by Iwaki et al. [2010], the 113 bp amplicon region in the VP2 Gene [Luo et al., 2009], the most stable region of the BKV genotype, was selected as the region of analysis. A total of 268 BKV DNA sequences were downloaded from the NCBI database and aligned using MEGA version 4.0 alignment/CLUSTALW [http://www.megasoftware.net; Kumar et al., 2008]. A neighbor-joining (NJ) tree view was generated using MEGA version 4.0. The results were used in the subsequent HRMA and DNA sequencing.
Real-Time PCR of BKV
The BKV samples examined in this study were selected from among those that had been sent to the study site (a laboratory) for routine clinical examination of cases of renal transplantation. Permission for the use of patient samples for BKV DNA analysis was granted by the Institutional Review Board (IRB)/Ethics Committee of the University of Southern California. No personally identifying data were associated with the samples to protect patient privacy in accordance with the Declaration of Helsinki [CIOMS/WHO, 2002a] and International Ethical, Guidelines for Biomedical Research Involving Human Subjects [CIOMS/WHO, 2002b].
DNA was extracted from 200 µl of uncentrifuged urine and plasma samples using the QIAamp blood mini kit (Qiagen, Valencia, CA). The final elution volumes were 100 µl of each urine sample and 60 µl of each plasma sample. Real-time PCR was performed to calculate the BKV load in urine and plasma samples using the primers and PCR protocol previously described by Iwaki et al. [2010]. Briefly, the Polyoma_4.2 (f) and BKV_5.1 (r) primer pair and the BKV-MGB probe were used in a thermal-cycling process consisting of (i) pre-incubation at 95°C for 10 min; (ii) performance of 45 cycles of a 3-cycle PCR, with each cycle consisting of incubation at (a) 95°C for 10 sec, (b) 58°C for 20 sec, (c) and 65°C for 1 sec; and (iii) cool-down by incubation at 40°C for 30 sec.
HRMA and DNA Sequencing
One amplicon from each from the three subgroups (GI-1, GI-2, and GII-IV) was purified from patient urine samples for use as a reference in the analysis. HRMA was performed on DNA obtained from 14 randomly selected urine samples using the Light Cycler 480 instrument (Roche Applied Science, Indianapolis, IN). Heteroduplexes for HRMA were formed in a total reaction volume of 12 µl comprised of 5 µl of amplified PCR sample product, 5 µl of the reference sample PCR amplicon, 0.7 µl of LightCycler® 480 ResoLight Dye (Roche Applied Science), 0.5 µl of high salt solution (1.0 M KCl, 0.5 M Tris-HCl at pH = 8.0), and 0.8 µl of distilled water. The value of the fluorescence intensity of the quantitative real-time PCR was used to calculate the volume of PCR product required for mixing. To induce heteroduplex formation, the DNA mixtures were heated at 95°C for 10 sec before being cooled to 60°C for 10 sec at a rate of 20°C/sec. The heteroduplex DNA fragments were melted from 60 to 90°C at a rate of 0.1°C/sec. The melting profiles were analyzed with Gene Scanning software (Roche Applied Science) according to a two-step procedure. First, the fluorescence curves of samples and references were horizontally normalized to approximate their 79.0–80.0°C pre-melt temperatures and then to approximate their 86.0–87.0°C post-melt temperatures. Second, the normalized fluorescence curves were vertically shifted and overlaid to superimpose them on approximately 9–15% of the fluorescence value of each curve.
To validate HRMA performance, the conventional method of DNA sequencing was performed for the same samples. Briefly, the amplified PCR product from each sample was purified using a Qiagen PCR purification kit (Qiagen). The purified DNA was then sequenced by conventional means using the BigDye Terminator Cycle Sequencing Ready Reaction Kit Version 3.1 on an ABI 377 Sequencer (Applied Biosystems, Foster City, CA). Using this method, the categorization of PCR products by HRMA was verified via conventional DNA sequencing of the14 urine samples selected at random. Further validation was performed using 49 samples selected from among 211 samples taken from patients who had undergone transplantation surgery between 2008 and 2009. The criteria for sample selection were availability of urine and serum samples on the same date and detection of a positive BKV load in at least the urine sample.
Statistical Analysis
The correlation between the BKV load in plasma and urine was determined by performing non-parametric Spearman rank correlation testing. The categorical variables of BKV load in each genotype category were compared by performing Fisher's exact testing. All analyses were performed using Dr. SPSS II for Windows (SPSS, Chicago, IL).
RESULTS
Using the method of phylogenetic analysis described by Luo et al. [2009], the BKV strains were categorized into three genotypes depending on the common sequences identified in the amplicons, as shown in Fig. 1a and b. Group GI-1 included the subtype I subgroups Ia, Ib1, and Ic, into which 137 strains were categorized. Group GI-2 included only the subtype I subgroup Ib2, into which 48 strains were categorized. Group GII-IV included the subtypes II, III, and IV, into which 80 strains were categorized. The categorization of each BKV strain into the genotype group subtype II, III, or IV depended on the SNPs detected in the unselected sequence, as described by Luo et al. [2009]. However, the MM (V01109.1; MM), CAP-h2 (AY628226.1; Caph2), and Pitt VR-10 (DQ989808.1; Pitt10) strains could not be categorized into one of these three genotype groups because of a specific characteristic. Specifically, the MM strain was categorized into subtype Ia but had an additional 1558 A > G SNP, the Pitt10 strain was categorized into subtype Ib2 but had an additional 1580 G > A SNP, and the Caph2 strain was categorized into subtype Ib1 but had an additional 1573 A > G SNP.
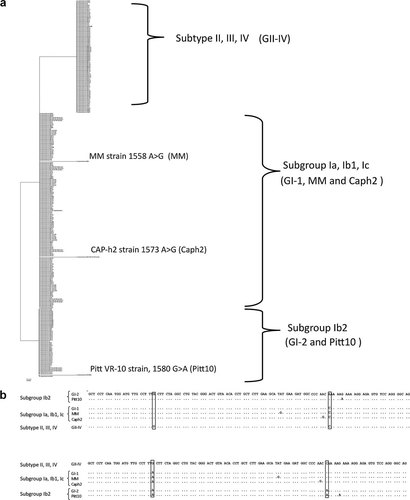
a: Phylogenetic analysis tree view constructed by the NJ method using MEGA version 4.0 software. BK polyomavirus (BKV) genotypes were classified into three genotypes: GI-1 comprised subtype I subgroups Ia, Ib1, and Ic; GI-2 comprised subtype I subgroup Ib2; and GII-IV comprised subtypes II, III, and IV. These three genotypes were used for the real-time PCR amplicon. MM strain belonged to subtype Ia, but had an additional SNP of 1558 A > G (MM), Pitt VR-10 strain belonged to the subtype Ib2, but had an additional SNP of 1580 G > A (Pitt VR10), and CAP-h2 strain belonged to the subtype Ib1, but had an additional SNP of 1573 A > G (Caph2). b: Amplicon sequence alignment. Upper sequences are GI-2 strain reference alignment, and alphabets denote the nucleotide difference. Lower sequences are GII-IV strain reference alignment, and alphabets denote the nucleotide difference.
Figure 2 shows 3 different patterns of melting curves resulting from HRMA analysis of the 14 urine samples, 5 of which were categorized as the GI-1 strain, 5 as the GI-2 strain, and 4 as the GII-IV strain, with the GI-2 and GII-IV strains serving as references. As can be observed in Fig. 2b and d, those strains with one or two mismatches demonstrated one or two melting curve peaks, respectively, relative to the references when analyzed with Gene Scanning software after fluorescence normalization and superimposition of the temperature overlay on the melting curves.
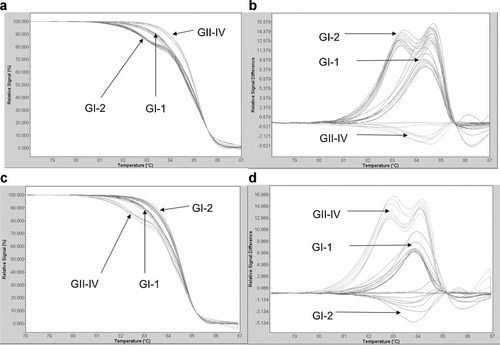
Fourteen BK polyomavirus (BKV) samples were genotyped using high-resolution melt analysis (HRMA) in the LightCycler 480 (Roche Applied Science). The genotypes of the 14 samples, as determined by the HRMA method were then validated by conventional DNA sequencing using the ABI PRISM® 377 DNA sequencer (Applied Biosystem). a: Normalized and temperature shifted melting curve with GII-IV strain reference. b: Fluorescence difference plot between samples and GII-IV strain reference. c: Normalized and temperature shifted melting curve with GI-1 strain reference. d: Fluorescence difference plot between samples and GI-1 strain reference.
The categorization of the 14 DNA samples into 3 groups by HRMA was then validated using conventional DNA sequencing methods. The DNA sequencing results indicated that 5 samples should be categorized into group GI-1, 5 into group G1-2, and 4 into group GII-IV. To compare the GI (GI-1 and GI-2) and GII-IV genotype groups, the analysis results of all 49 patient samples were reviewed. As can be observed in Fig. 3, which shows the range of BKV concentrations obtained from the 49 samples, 20 samples could be categorized into group GI-1, 18 into group GI-2, and 11 into group GII-IV, suggesting that 77.6% (38/49) of the samples were positive for the GI genotype.
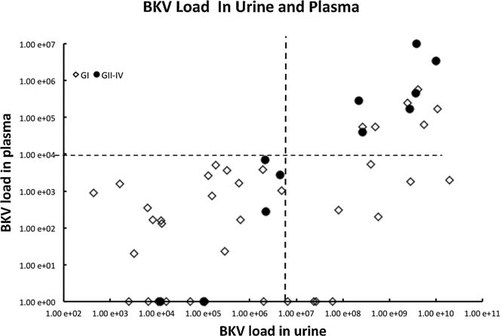
BK polyomavirus (BKV) load value in urine and plasma in 49 patients ⋄: GI (GI-1 and GI-2) and ●: GII-IV.
Plasma BKV load was found to be significantly higher in group GII-IV than group GI (P = 0.016, Fisher's exact testing; odds ratio [OR] = 6.4). Figure 2 shows the results of comparison of BKV load measured in urine and plasma using a cut-off level of 107 copies/ml for urine and 104 copies/ml for plasma. Of the 38 patient samples classified as group GI, 6 could be classified as GI when the cut-off levels were applied. Of the 11 patient samples classified as group GII-IV, 6 appeared in the area above the cut-off levels in both the urine and plasma samples when the cut-off levels were applied. The difference in the ratio of number of samples above the cut-off levels was found to be statistically significant (P = 0.016; Fisher's exact testing). Moreover, a significant positive correlation was found between urine and plasma BKV load in group GII-IV (P < 0.001; coefficient of correlation = 0.934), as well as in group GI. However, the correlation between urine and plasma BKV load was found to be weaker in cases testing positive for group GI compared to group GII-IV (P = 0.001; coefficient of correlation = 0.502; Fig. 3).
DISCUSSION
From analysis of sequence alignment data, a theoretical model of BKV categorization and method of HRMA assay were developed to differentiate among three BKV strain genotypes using two different reference DNA strains. The results of this sequence alignment of 113 bp amplicon using MEGA 4.0 software demonstrated that more than 250 BKV variants could be categorized into three genotype groups (GI-1, GI-2, and GII-IV) and three strains (MM, Caph2, and Pitt10) with a high level of sensitivity in an efficient manner, as confirmed by conventional DNA sequencing.
The HRMA method described here is based on analysis of a fluorescence curve reflecting the intensity profile of intercalated fluorescence dye induced by continuous temperature increases. Several factors that affect the accuracy of the results, including the extent of the mismatching of heteroduplex DNA double-strand formation and the ratio of each DNA strain used, lead to the production of different melting curve profiles useful in the detection of SNPs in the amplicon. The number and positioning of nucleotide mismatching of the sample amplicon and the ratio and concentration of the sample amplicon against the reference amplicon sequence are two particularly important factors to consider in devising an HRMA method to screen pathogenic DNA variants. Specifically, the ratio of the sample strain to the reference strain (amplicon) must be adjusted to 1:1 in each sample tested.
As shown Fig. 2, a DNA strain with multiple mismatches can be clearly delineated using HRMA. However, it is difficult to differentiate among DNA strains with the same number of mismatches but in which the mismatches occur in different positions using HRMA. For example, Hung et al. [2009] found that one SNP demonstrated the same fluorescence curve as that of the wild type of the FBN1 gene when they used HRMA [Hung et al., 2009]. Compared to the GI-2 strain, the GI-1 and Pitt10 strains had one mismatch, while the GII-IV, MM, and Caph2 strains had two mismatches at a given sequence. In comparison to the GII-IV strain, the GI-1 had 1 mismatch; the GII-IV, MM, and Caph2 strains had two mismatches; and the Pitt10 strain had three mismatches at a given sequence, as shown in Fig. 1b. By employing both the GI-2 and GII-IV amplicons as BKV references, it was relatively easy to distinguish among the GI-1, GI-2, and GII-IV genotypes, as shown in Fig. 2.
Theoretically, analysis of the different high-resolution melting curve patterns resulting from an additional number of SNPs at a given region, as shown in Fig. 1b, allows for differentiation among the BKV strains MM, Caph2, and Pitt10 using HRMA. However, in practice, it is difficult to differentiate the MM from the Caph2 strain because both have the same number of SNPs and the same extent of SNP variation, even at different sites of mismatch in the amplicon. Despite this limitation, the HRMA method described here yields results within 5 hr with a high level of sensitivity and specificity, making it more efficient than conventional DNA sequencing in terms of cost and time. As BKV is characterized by remarkable diversity, which results from the many genomic mutations in its sequence, it is important to choose a highly stable region that includes BKV genotype-related SNPs for amplification. As had Iwaki et al. [2010] in their design of real-time PCR primers, a region of BKV with a low rate of mutations was selected for BKV genotype analysis in this study.
BKV subtype I (genotyped as GI in this study) with an archetypical non-coding control region (NCCR) has been reported as the most prevalent subtype [Jin et al., 1993; Hirsch and Steiger, 2003]. In a study of the effects of genomic mutations of the VP1 region of BKVN, Boldorini et al. [2009b] found the GI strain to be the predominant BKV strain (Boldorini et al., 2009b). In agreement with previous studies [Jin et al., 1993; Hirsch and Steiger, 2003; Boldorini et al., 2009a], 77.6% (38/49) of the samples in this study could be categorized into the GI genotype group. However, unlike in previous studies, the MM, Pitt VR-10, and Caph2 strains were not detected. These strains should have other characteristic melting curve profiles, as discussed above.
Accurate diagnosis of BKVN requires histological examination of the renal biopsy sample [Bonvoisin et al., 2008]. As renal biopsy data were unavailable when the present study was conducted, they could not be examined to verify the accuracy of the results. Therefore, the existence of a positive correlation between the risk of BKVN and the presence of BKV genotypes cannot be concluded solely from the findings of this study. Nevertheless, the findings of (i) a significantly higher incidence of risk markers of serum BKV load in the GII-IV group and (ii) a significantly positive correlation between BKV load in urine and plasma are interesting and potentially significant. Moreover, the identification of a correlation between BKV load in the urine and plasma of a GII-IV genotype—rather than the more expected existence of a correlation between BKV load in the urine and plasma of a GI genotype—is both surprising and potentially significant.
The HRMA method developed and presented in this study using two different references offers a simple, efficient, and effective procedure for the identification of SNPs at the RT-PCR amplification region of BKV. Building on the findings of this study, similar studies using a larger cohort of patients should endeavor to improve the resolution of strain identification and validate the use of HRMA as a diagnostic tool in determining the risk of BKVN in transplant patients. As more evidence of its efficacy is accumulated, practitioners will gain increasing confidence in using this method to obtain the most accurate clinical data possible with which to predict the prognosis of their renal transplantation patients.




