Molecular characterization of rotavirus strains from children with diarrhea in Italy, 2007–2009†
The work was designed and performed at the Istituto Superiore di Sanità, coordinating the activities of regional partners of the RotaNet-Italy Study Group.
Abstract
The surveillance network RotaNet-Italia was established in 2007 in order to investigate the diversity of co-circulating rotavirus strains in Italy, and to provide a baseline for future assessment of possible effects of vaccine implementation in selecting novel versus common rotavirus strains. A total of 2,645 rotavirus strains from pediatric patients with acute diarrhea were collected over three consecutive seasons from September 2006 through August 2009, and partially characterized by standardized multiplex RT-PCR. Most of strains (89.1%) belonged to genotypes G1–G4, and G9, associated with either P[8] or P[4], commonly found in humans worldwide. However, in at least 2.0% of cases, viruses exhibited either a G or P type typical of animal viral strains, suggesting gene reassortment events between rotaviruses of different origin. Mixed infections with two or more rotavirus strains were observed frequently (7.6% of patients), and depended on the frequencies of co-circulating rotaviruses of one particular genotype. The numbers and genotypes of likely natural reassortants of common genotype rotaviruses were found to be correlated with the observed numbers and genotypes of mixed infections. Large variation in the relative frequency of different rotavirus genotypes was observed between different seasons and/or areas of Italy, suggesting independent evolution or differential introduction of viral strains with respect to both time and space. J. Med. Virol. 83:1657–1668, 2011. © 2011 Wiley-Liss, Inc.
INTRODUCTION
Rotaviruses cause a large proportion of acute gastroenteritis cases in infants and young children worldwide, with a death toll of more than 500,000 yearly, mostly in developing countries [Parashar et al., 2006]. Each year, rotaviruses cause approximately 111 million episodes of acute gastroenteritis requiring home care, 25 million clinic visits, and 2 million hospitalizations, altogether constituting a major economic burden for health care systems and families in both USA and European countries [Parashar et al., 2006; Soriano-Gabarro et al., 2006; Forster et al., 2009]. These figures are consistent with the need for implementing vaccination strategies in both developing and developed countries, as recommended by the World Health Organization [WHO, 2007].
Two rotavirus vaccines have been licensed in Europe, USA, and other countries throughout the world, and have shown high efficacy in phase III clinical trials [Ruiz-Palacios et al., 2006; Vesikari et al., 2006b]. These are: (i) the live attenuated monovalent human rotavirus vaccine Rotarix (GlaxoSmithKline, London, England) constituted by a single G1P1A[8] strain [Ruiz-Palacios et al., 2006]; (ii) the live attenuated pentavalent human-bovine reassortant vaccine RotaTeq [Clark et al., 2006; Vesikari et al., 2006b] presenting human serotype G1 through G4, and P1A[8] specificities (Merck, Whitehouse Station, NJ). A remarkable decline of pediatric admissions for rotavirus diarrhea was reported in Philadelphia in 2008, and attributed to specific herd immunity consolidated by the increasing vaccine use during the previous 2 years [Clark et al., 2009]. Although this study noted that most of 2008 cases were infected with genotype G3 rotavirus, their low number allowed no inference on a possible differential efficacy of vaccination by viral serotype. Nonetheless, a high efficacy (95%) of the pentavalent vaccine towards G3P[8] infection was later demonstrated in Texas [Boom et al., 2010]. Further studies will likely generate more data, particularly in the Americas, where children immunization has been adopted on a larger scale than elsewhere [Parashar et al., 2006; de Oliveira et al., 2008].
The correlates of protection from rotavirus disease are not fully defined [Desselberger and Huppertz, 2011], but it is largely accepted by the scientific community that both outer capsid proteins of rotavirus VP7 and VP4 stimulate neutralizing antibody responses [Yuan et al., 2009], which may be important for forming complexes with the virus on the intestinal mucosa [Coffin et al., 1997; To et al., 1998] or prevent viremia and possible extra-intestinal rotavirus replication [Blutt et al., 2007].
The issue of protective immunity from rotavirus infection is related to the complexity of the virion, exhibiting three protein layers and six structural proteins, and of its genome consisting of 11 double-stranded (ds) RNA segments which encode 11 or 12 proteins [Pesavento et al., 2006]. The ability of rotavirus to exchange entire genes during multi-strain infection of cells (reassortment) is known since many years [Gombold and Ramig, 1986; Offit and Blavat, 1986], is observed during infection in vivo in humans and animals [Iturriza-Gomara et al., 2001; Watanabe et al., 2001; Maunula and Von Bonsdorff, 2002; Esona et al., 2009], and is a major cause of the exceptional genetic variability observed for clinical rotavirus strains worldwide [Santos and Hoshino, 2005]. Interspecies rotavirus reassortment may have implications for vaccine programs effectiveness because it may introduce viral serotypes typical of animals into potentially naïve human populations, independent of vaccine coverage [Gentsch et al., 2005]. The second major factor in rotavirus genetic diversity is the accumulation of point mutations generated by the error-prone RNA-dependent RNA polymerase, that can generate neutralization escape variants [Shaw et al., 1988; Ciarlet et al., 1997; Iturriza-Gomara et al., 2001; Arista et al., 2005] and misrecognition of viral genes by established genotyping primers [Santos et al., 2003; Parra and Espinola, 2006; Solberg et al., 2009].
Despite the large diversity among rotavirus strains, a broad cross-reactive immunity between viral serotypes in vitro and in vivo is supported by several studies [Gorrell and Bishop, 1999; Yuan et al., 2005; Vesikari et al., 2007], and may explain the reported efficacy of vaccines against circulating wild-type rotaviruses of G- and P-types which are not encompassed in vaccine formulations.
To track rotavirus genes evolution, zoonotic interchange and reassortment of group A rotaviruses [Matthijnssens et al., 2008a; Martinez-Laso et al., 2009; Song and Hao, 2009], a recently proposed new classification scheme considering all 11 genome segments [Matthijnssens et al., 2008a,b] has been very useful. Based on extensive genome sequencing, this system is suited for very specific studies on rotavirus phylogeny or for detailed investigations on uncommon strains emerging in the population. Multiplex RT-PCRs with diverse primer sets are normally used for characterizing fecal group A rotavirus strains into G- and P-types, relative to the genes encoding capsid (Glycosylated) VP7 and (Protease-sensitive) VP4 proteins [Gouvea et al., 1990; Gentsch et al., 1992; Iturriza-Gomara et al., 2004; Simmonds et al., 2008]. By applying this dual nomenclature method [Estes and Kapikian, 2007; Desselberger et al., 2009], at least 23 G-genotypes and 32 P-genotypes were differentiated [Trojnar et al., 2009; Ursu et al., 2009; Collins et al., 2010], including 10 G- and 11 P-types in human infections, respectively [Jayaram et al., 2004; Desselberger et al., 2009]. Molecular typing methods have improved the understanding of rotavirus strain diversity, and permitted establishment of large molecular epidemiology programs [Gentsch et al., 2005; Santos and Hoshino, 2005; Iturriza-Gomara et al., 2009, 2010], which will be valuable to control the efficacy of present vaccines towards different co-circulating wildtype rotaviruses, estimate the impact of possible vaccine-driven immune selection of wildtype strains and help shaping possible future rotavirus vaccines development [Desselberger et al., 2006].
To gather comprehensive information on co-circulating rotavirus genotypes throughout Europe, a large consortium was established in 2007 [Iturriza-Gomara et al., 2009], embracing 17 countries, using shared methods and viral databases.
The present study reports the molecular characterization of rotaviruses from hospitalized children with diarrhea during a 3-year surveillance activity in Italy, and discusses temporal and geographical differences in the incidence of infection with different genotypes.
MATERIALS AND METHODS
RotaNet-Italy Study Group
A laboratory-based network was established by the Istituto Superiore di Sanità in 2007, in collaboration with the Ministry of Health, Regional Public Health Services, Hospitals and Universities in different Italian regions. Table I lists the collaborating institutions and the number of rotavirus strains contributed. Regional centers were selected to ensure a large pediatric patients catchment area across the country and to broaden the capacity of laboratory rotavirus diagnosis.
| Region | Rotavirus strain number, by yeara | |||
|---|---|---|---|---|
| 2007 | 2008 | 2009 | Total | |
| Piedmont | 100 | 133 | 44 | 277 |
| Liguria | 22 | — | 22 | |
| Lombardia | 59 | 255 | 314 | |
| Veneto | 31 | 358 | 132 | 521 |
| Total Northern regions | 131 | 572 | 431 | 1,134 |
| Emilia-Romagna | 53 | 155 | 120 | 328 |
| Marche | 63 | 58 | 121 | |
| Tuscany | 17 | 176 | 35 | 228 |
| Umbria | 53 | 89 | 55 | 197 |
| Lazio | 19 | 31 | 50 | |
| Total Central regions | 123 | 502 | 299 | 924 |
| Calabria | 17 | 33 | 40 | 90 |
| Campania | 4 | 41 | 45 | |
| Apulia | 180 | 73 | 87 | 340 |
| Sicily | 69 | 95 | 164 | |
| Total Southern regions | 197 | 179 | 263 | 639 |
| All regions | 451 | 1,253 | 993 | 2,697 |
- a Rotavirus year is the 12-month period containing the winter–spring infection peak, from September of the previous calendar year through August of designated year.
Patients and Samples
Stool samples were collected from children aged less than 5 years, admitted with acute gastroenteritis to hospitals or outpatient wards between 2006 and 2009 seasons. According to the European network, a rotavirus season was defined as the 12-month period from September through the end of August of the following year. Each specimen was labeled with a unique surveillance identification number. Epidemiological and patient data (age, sex, geographical location, date of onset, date of sample collection, and symptoms) were recorded using ad hoc questionnaires, and entered into a database for linkage to genotyping data. Patients' privacy and confidentiality issues were managed in agreement with national legislation.
Rotavirus Testing
Stools were collected at admission, analyzed right after collection or stored at −20°C until processing. Rotavirus screening was performed with commercial tests validated in each laboratory, including immuno-chromatographic, immuno-enzymatic, and latex-agglutination formats. After rotavirus identification by local laboratories, positive stools were shipped in dry ice to the Istituto Superiore di Sanità in Rome, for strain characterization, maintaining the cold-chain until genotyping. For molecular testing, 10% suspensions in water, were clarified by micro-tube centrifugation (15,700g for 15 min) and used for rotavirus-specific reverse transcription (RT)-PCR and nested PCR.
Nucleic Acid Extraction and G/P Rotavirus Typing
Rotavirus dsRNA was extracted from 140 µl of the 10% fecal suspension by either QIAamp Viral RNA Mini Kit (Qiagen, Milan, Italy) or SV Total RNA Isolation System (Promega, Milan, Italy), according to the manufacturer's instructions. RNA was eluted in 40 µl of RNase-free water, and stored at −80°C or used immediately. G and P rotavirus genotyping was performed by standardized RT-PCR methods (http://www.who.int/nuvi/rotavirus/WHO_IVB_08.17_eng.pdf; http://www.eurorota.net/docs.php). Essentially, for identification of G type, VP7-F and VP7-R consensus primers were used in first-round PCR. Subsequently, the VP7-R primer was used in a nested multiplex PCR together with the G1-G4, G8-G10, and G12-specific forward primers. Con2–Con3 consensus primers were used in RT-PCR for P typing, followed by standard multiplex PCR including the Con3 primer, in combination with type-specific primers for P4, and P6-P11. When necessary, rotavirus infection was confirmed by VP6-specific RT-PCR [Gouvea et al., 1990; Gentsch et al., 1992; Iturriza-Gomara et al., 2004]. For genotype assignment, the molecular size of PCR amplicons, separated by agarose gel electrophoresis, was determined using a Molecular Imager Gel Doc XR with the Quantity-One software (BioRad, Segrate, Italy). The performance of the laboratory is evaluated annually by a genotyping Ring Test on blind samples administered within the EuroRotaNet circuit.
DNA Sequencing and Analysis
A total of 53 rotavirus strains were confirmed by nucleotide sequencing of PCR amplicons. These included a random selection of “common” strains to control the reliability of the genotyping PCR, samples exhibiting an uncommon or ambiguous genotype, and a fraction of amplicons suggesting mixed genotype infections. Gene 4 or gene 9 DNA bands were purified using Wizard SV Gel and PCR Clean-Up System (Promega), and sequenced using the PCR primers with the BigDye Terminator Cycle Sequencing Ready Reaction Kit version 3.1 (Perkin Elmer, Applied Biosystems, Foster City, CA) in an automated sequencer (ABI Prism 310 DNA sequencer, Applied Biosystems). Sequences obtained were compared against the NCBI GenBank database (http://www.ncbi.nlm.nih.gov), using the DNASIS Max v.2 software (Hitachisoft, Hamburg, DE).
Analysis of Mixed Infections and Natural Reassortants
The numbers of observed likely natural reassortants G1P[4], G2P[8], G3P[4], G4P[4], and G9P[4] of the seasons 2008 and 2009 were compared with the numbers of relevant mixed infections observed during this time period, using linear regression analysis.
RESULTS
Sampling of Rotavirus Strains in Italy
Rotavirus cases, stool, and data reporting to the central laboratory were implemented in Italy since late 2006, under a surveillance project (2007) coordinated by the Istituto Superiore di Sanità and supported by the Ministry of Health. The project is linked to the EuroRotaNet consortium, and enrolls hospitals and laboratories spread across Italy.
Rotaviruses were identified in 2,697 stools of children, requiring hospitalization or laboratory testing for acute gastroenteritis, during three consecutive years, including winter-spring peaks of 2007–2009. The numbers of rotavirus strains collected by region is reported in Table I, and overall increased from 451 in 2007 (7 of 20 Regions) to 1,253 and 993 in 2008 and 2009 (13 Regions), respectively.
Prevalence of Rotavirus G and P Types
Out of 2,697 rotaviruses detected by routine diagnostic, 2,645 were confirmed by RT-PCR genotyping (Table II). Among the VP7 genotypes identified, G1 predominated in Italy during the entire period accounting for approximately 45% (range 38.8–48.8%) of strains investigated, followed by G9 (22.4%), G4 and G2 (10.2 and 8.5%, respectively). G3 Rotaviruses were observed in 3.1% of cases, being rare in 2007. Major variation between years was also observed for G2P[4] strains, which ranged from 17.0% in 2007 to 6.2% in 2009, and G4P[8] (11.8% in 2007; 6.1% in 2008; 14.7% in 2009). In most specimens, common G-types, (G1, G3, G4, and G9, and G2) were associated with common P[8] and P[4], respectively, comprising on average 89.1% of all fully G- and P-typed specimens.
| Genotype | Number of genotyped strains (%), by yeara | |||
|---|---|---|---|---|
| 2007 | 2008 | 2009 | 2007–2009 | |
| G1P[8] | 212 (47.3) | 598 (48.8) | 377 (38.8) | 1,187 (44.9) |
| G2P[4] | 76 (17.0) | 88 (7.2) | 60 (6.2) | 224 (8.5) |
| G3P[8] | 2 (0.4) | 45 (3.7) | 35 (3.6) | 82 (3.1) |
| G4P[8] | 53 (11.8) | 75 (6.1) | 143 (14.7) | 271 (10.2) |
| G9P[8] | 74 (16.5) | 285 (23.3) | 233 (24.0) | 592 (22.4) |
| Common genotypes | 417 (93.1) | 1,091 (89.1) | 848 (87.2) | 2,356 (89.1) |
| Uncommon | 1 (0.2) | 20 (1.6) | 31 (3.2) | 52 (2.0) |
| Mixed types | 22 (4.9) | 106 (8.7) | 72 (7.4) | 200 (7.6) |
| Untypable | 8 (1.8) | 8 (0.7) | 21 (2.2) | 37 (1.4) |
| Total | 448 (100.0) | 1,225 (100.0) | 972 (100.0) | 2,645 (100.0) |
- a Rotavirus year is the 12-month period containing the winter–spring infection peak, from September of the previous calendar year through August of designated year.
Unconventional G and P associations, particularly G1, G4 or G9 with P[4] (6, 3, and 8 strains, respectively), and G2P[8] (8 cases), or uncommon rotavirus genotypes, such as G10P[8] (15 strains) and G12P[8] (1 case), were observed in 2.0% of cases (Table III). In further 10 cases, other unusual human G or P alleles were detected, such as G8, P[6], and P[9], in combination with common human P or G types.
| Genotype | Number of strains, by yeara | |||
|---|---|---|---|---|
| 2007 | 2008 | 2009 | 2007–2009 | |
| G1P[4] | — | 6 | — | 6 |
| G2P[8] | — | 5 | 3 | 8 |
| G4P[4] | — | 2 | 1 | 3 |
| G9P[4] | — | 2 | 6 | 8 |
| G1P[6] | — | — | 2 | 2 |
| G3P[6] | — | — | 7 | 7 |
| G8P[8] | — | — | 1 | 1 |
| G9P[9] | — | 1 | — | 1 |
| G10P[8] | — | 4 | 11 | 15 |
| G12P[8] | 1 | — | — | 1 |
| Total | 1 | 20 | 31 | 52 |
- a Rotavirus year is the 12-month period containing the winter–spring infection peak from September of the previous calendar year through August of designated year.
Altogether, mono- (Table III) or mixed (Table IV) infections with a P[6] rotavirus were detected in 17 of 2,645 patients (0.7%). All seven G3P[6] strains (Table III) were found in Apulia (Southern Italy) in 2008–2009, suggesting a geographically limited circulation of this strain. The two G1P[6] strains identified (Table III) originated from Sicily (Southern Italy) and Emilia-Romagna (North-central), in 2009. The eight mixed P[6] infections were recorded in Emilia-Romagna mostly in 2009 (Table IV).
| G-P detected | Yearc | Probable rotavirus involved | ||||
|---|---|---|---|---|---|---|
| 2007 | 2008 | 2009 | Total | Cases | Types | |
| G1,2 P[4,8] | 1 | 5 | 9 | 15 | 38 | G1P[8], G2P[4] |
| G1,2 P[4] | 14 | 4 | 18 | |||
| G1,2 P[8] | 3 | 3 | ||||
| G1 P[4,8] | 2 | 2 | ||||
| G1 P[6,8] | 1 | 1 | 1 | G1P[8], G?P[6] | ||
| G1,3 P[8] | 1 | 1 | 1 | G1P[8], G3P[8] | ||
| G1,4 P[8] | 2 | 6 | 5 | 13 | 13 | G1P[8], G4P[8] |
| G1,9 P[8] | 18 | 43 | 7 | 68 | 68 | G1P[8], G9P[8] |
| G2,3 P[4,8] | 1 | 1 | 3 | G2P[4], G3P[8] | ||
| G2,3 P[4] | 2 | 2 | ||||
| G2 P[4,6] | 1 | 1 | 1 | G2P[4], G?P[6] | ||
| G2,4 P[4,8] | 2 | 2 | 11 | G2P[4], G4P[8] | ||
| G2,4 P[4] | 7 | 1 | 8 | |||
| G2,4 P[8] | 1 | 1 | ||||
| G2,9 P[4,8] | 8 | 12 | 20 | 27 | G2P[4], G9P[8] | |
| G2,9 P[4] | 2 | 2 | ||||
| G9 P[4,8] | 1 | 3 | 1 | 5 | ||
| G2 P[4,8] | 1 | 1 | 1 | G2P[4], G?P[8] | ||
| G4,9 P[8] | 5 | 3 | 8 | 8 | G4P[8], G9P[8] | |
| G9 P[6,8] | 1 | 1 | 1 | G9P[8], G?P[6] | ||
| G1,2 P[4,6] | 1 | 1 | 2 | 2 | G1P[?]a, G2P[4], G?P[6]a | |
| G1,2,4 P[8] | 3 | 3 | 3 | G1P[8], G2P[4], G4P[8] | ||
| G1,2,9 P[4,8] | 1 | 1 | 17 | G1P[8], G2P[4], G9P[8] | ||
| G1,9 P[4,8] | 5 | 11 | 16 | |||
| G2,4 P[6,8] | 1 | 1 | 1 | G2P[4], G4P[8], G?P[6] | ||
| G2,9 P[4,6] | 1 | 1 | 1 | G2P[4], G9P[8], G?P[6] | ||
| G3 P[4,6] | 1 | 1 | 1 | G2P[4], G3P[?]b, G?P[6]b | ||
| G3,4 P[4,8] | 1 | 1 | 1 | G2P[4], G3P[8], G4P[8] | ||
| G4,9 P[4,8] | 1 | 1 | 1 | G2P[4], G4P[8], G9P[8] | ||
| Total | 22 | 105 | 65 | 192 | 192 | |
- a Possible G1P[6] reassortant strain.
- b Possible G3P[6] reassortant strain.
- c Rotavirus year is the 12-month period containing the winter-spring infection peak from September of the previous calendar year through August of designated year.
Mixed Rotavirus Infections
Infections with more than one type of rotavirus were suggested by co-amplification of multiple bands. Of 200 mixed infection cases (200/2,645; 7.6%), 170 and 22 involved two or three of the common (G1–G4, G9) strains (Table IV). Simultaneous presence of uncommon P[6] with P[4] (4 cases) or P[8] (2 cases) types, or both (2 cases), and one or two of G1-G4, or G9 G-types (Table IV) occurred in 8 cases.
Among the 170 double rotavirus infections (Table IV), the G1 + G9P[8] combination occurred most commonly (68 stools; 40.0%). Detection of G1 and/or G2, and P[4] and/or P[8], suggested co-infection with G2P[4] and G1P[8] rotaviruses in 38 (22.4%) cases.
Similarly, presence of specific combinations of amplified DNA bands (including all or only part of the parental strain G- and P-bands; see Table IV) identified G2P[4] and G9P[8] co-infections in 27 cases (15.9%), and G2P[4] and G4P[8] in 11 (6.5%). Double infections with G1P[8]–G4P[8] (13 cases; 7.6%), and G4P[8]–G9P[8] strains (8 cases; 4.7%) were also frequent.
Triple infection with common rotavirus genotypes (Table IV), involved mostly G2P[4], G1P[8], and G9P[8] strains (17/22; 77.3%).
The frequencies of individual “common” rotavirus infections were compared to the frequencies of observed infections with specific “mixed” genotypes. Table V lists the numbers of mixed infections with the five “common” genotypes observed during the years 2007–2008 and 2008–2009, for which the numbers of mixed infections were reasonably high. A correlation of low significance (P < 0.05) was found between the numbers and genotypes of mixed infections and the numbers of emerged likely natural reassortants. However, given the relatively small numbers of natural reassortants in this clinical specimens collection, this may not necessarily be the case.
| Mixed infections detected | Mixed infections as possible source for natural reassortants detected | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genotypesa | No.b | G1P[4] | G2P[8] | G3P[4] | G4P[4] | G9P[4] | ||||||
| 2008 | 2009 | 2008 | 2009 | 2008 | 2009 | 2008 | 2009 | 2008 | 2009 | 2008 | 2009 | |
| G1,2P[4,8] | 5 | 9 | 5 | 9 | 5 | 9 | ||||||
| G1,2P[4] | 14 | 4 | 14 | 4 | ||||||||
| G1,2P[8] | 3 | 3 | ||||||||||
| G1P[4,8] | 2 | 2 | ||||||||||
| G2,3P[4,8] | 1 | 1 | 1 | |||||||||
| G2,3P[4] | 2 | 2 | ||||||||||
| G2,4P[4,8] | 2 | 2 | 2 | |||||||||
| G2,4P[4] | 7 | 1 | 7 | 1 | ||||||||
| G2,4P[8] | 1 | 1 | ||||||||||
| G2,9P[4,8] | 8 | 12 | 8 | 12 | 8 | 12 | ||||||
| G2,9P[4] | 2 | 2 | ||||||||||
| G9P[4,8] | 3 | 1 | 3 | 1 | ||||||||
| G2P[4,8] | 1 | 1 | ||||||||||
| G1,2P[4,6] | 1 | 1 | 1 | 1 | ||||||||
| G1,2,4P[8] | 3 | 3 | ||||||||||
| G1,2,9P[4,8] | 1 | 1 | 1 | 1 | ||||||||
| G1,9P[4,8] | 5 | 11 | 5 | 11 | 5 | 11 | ||||||
| G2,4P[6,8] | 1 | 1 | ||||||||||
| G2,9P[4,6] | 1 | 1 | ||||||||||
| G3P[4,6] | 1 | 1 | ||||||||||
| G3,4P[4,8] | 1 | 1 | 1 | |||||||||
| G4,9P[4,8] | 1 | 1 | 1 | |||||||||
| Total | 28 | 25 | 18 | 29 | 1 | 4 | 8 | 4 | 17 | 28 | ||
| No. of natural reassortants detected | 6 | 0 | 5 | 3 | 0 | 0 | 2 | 1 | 2 | 6 | ||
- If total number of mixed infections/year is used as independent variable x, and the number of natural reassortants detected as dependent variable y, the following linear regression line is found: y = 0.14x + 0.17. The correlation coefficient r is 0.66, that is, for degree of freedom of 8 (=n − 2) the relationship is significant at P < 0.05.
- a The P[6] genotypes were not considered in this context, as they are of likely animal origin and thus, whilst being found in mixed infections, they are not a factor in the natural reassortment of common human rotavirus strains.
- b Values taken from Table IV.
Nucleotide Sequencing of Untypable Rotavirus Strains
In a total of 72 cases, the result of genotyping PCR was ambiguous because a clearly defined DNA band was not detected after the multiplex PCR. However, 35 of these rotavirus strains were eventually characterized by sequencing the first PCR amplicon. Except one case where a G10P[8] was present, the other 34 cases belonged to common G and P genotypes. In further 37 cases, strains could not be typed, because no definite sequence could be generated and analyzed, as a consequence of inefficient DNA yield at PCR. Scarce virus concentration in these stool samples is the most likely explanation for genotyping failure, because in 19 of these cases both G and P typing failed. However, it cannot be excluded that some samples might contain either unusual G or P types or mutations in the sequences targeted by PCR primers. In three cases with partial genotyping, the only gene determined belonged to a type uncommon in humans, that is, G10, P[9], or P[10].
Geographical Diversity in Rotavirus Genotype Distribution in Italy
The variation in RV genotypes distribution by year of collection and geographical area is shown in Table VI, clustering data according to Northern, Central, and Southern groups of Italian regions (see region grouping details in Table I). Overall, G1P[8] rotavirus strains were constantly less prevalent in Southern Italy (from 20.9 in 2006–2007 to 30.7% in 2007–2008), than in the remaining Regions (from 73.6 in the North during 2006–2007 to 42.1% in Central Italy, 2008–2009). G2P[4] strains showed higher prevalence by both year (Table II) and area (Table VI) in the South. In fact, compared to an overall 8.5%, this genotype represented 17.3% and 25.2% of all strains in Southern and Central regions (vs. 8.5% in the North) in 2006–2007, 18.8% of strains from Southern Regions versus 4.3% and 6.3% in North and Center in 2007–2008, whereas in 2008–2009 it ranged from 4.8% to 9.5% North-to-South. G3P[8] strains were relatively more constantly found in all regions (mean 3.1%), but were virtually absent in Italy in 2006–2007 (0.2%), while increasing in Southern Regions in 2007–2008 (8.0%). G4P[8] was generally infrequent during the central year of study (2.8–7.9% in the South and Center, respectively, vs. overall 10.2%), as was in 2006–2007 in North-Central Italy (2.3–3.3%). Conversely, it was the first or second most prevalent genotype in Southern Regions in both 2006–2007 (23.5%) and 2008–2009 (35.1%), dropping to 2.8% in the South during the middle year. In 2006–2007, G9P[8] rotaviruses showed a trend (10.1%, 6.5%, and 27.0% in Northern, Central, and Southern Italy, respectively) similar to G4P[8], occurring more constantly in the other years. Uncommon rotavirus genotypes occurred rarely in 2006–2007 (one case, 0.2%), subsequently ranging from 1.2% to 3.8%. Uncommon and untypable (Table VI) strains were relatively more common in Southern Italy.
| Genotype | Number of genotyped strains (%), by area | |||||||
|---|---|---|---|---|---|---|---|---|
| Northern | Central | Southern | All | |||||
| No. | % | No. | % | No. | % | No. | % | |
| 2006–2007 | ||||||||
| Common | ||||||||
| G1P[8] | 95 | 73.6 | 76 | 61.8 | 41 | 20.9 | 212 | 47.3 |
| G2P[4] | 11 | 8.5 | 31 | 25.2 | 34 | 17.3 | 76 | 17.0 |
| G3P[8] | — | — | — | — | 2 | 1.0 | 2 | 0.4 |
| G4P[8] | 3 | 2.3 | 4 | 3.3 | 46 | 23.5 | 53 | 11.8 |
| G9P[8] | 13 | 10.1 | 8 | 6.5 | 53 | 27.0 | 74 | 16.5 |
| Uncommon | — | — | — | — | 1 | 0.5 | 1 | 0.2 |
| Mixed | 7 | 5.4 | 4 | 3.3 | 11 | 5.6 | 22 | 4.9 |
| Untypable | — | — | — | — | 8 | 4.1 | 8 | 1.8 |
| Total | 129 | 100.0 | 123 | 100.0 | 196 | 100.0 | 448 | 100.0 |
| 2007–2008 | ||||||||
| Common | ||||||||
| G1P[8] | 283 | 50.8 | 261 | 53.0 | 54 | 30.7 | 598 | 48.8 |
| G2P[4] | 24 | 4.3 | 31 | 6.3 | 33 | 18.8 | 88 | 7.2 |
| G3P[8] | 14 | 2.5 | 17 | 3.5 | 14 | 8.0 | 45 | 3.7 |
| G4P[8] | 31 | 5.6 | 39 | 7.9 | 5 | 2.8 | 75 | 6.1 |
| G9P[8] | 155 | 27.8 | 92 | 18.7 | 38 | 21.6 | 285 | 23.3 |
| Uncommon | 9 | 1.6 | 6 | 1.2 | 5 | 2.8 | 20 | 1.6 |
| Mixed | 41 | 7.4 | 46 | 9.3 | 19 | 10.8 | 106 | 8.7 |
| Untypable | — | — | — | — | 8 | 4.5 | 8 | 0.7 |
| Total | 557 | 100.0 | 492 | 100.0 | 176 | 100.0 | 1,225 | 100.0 |
| 2008–2009 | ||||||||
| Common | ||||||||
| G1P[8] | 188 | 45.0 | 123 | 42.1 | 66 | 25.2 | 377 | 38.8 |
| G2P[4] | 20 | 4.8 | 15 | 5.1 | 25 | 9.5 | 60 | 6.2 |
| G3P[8] | 17 | 4.1 | 11 | 3.8 | 7 | 2.7 | 35 | 3.6 |
| G4P[8] | 30 | 7.2 | 21 | 7.2 | 92 | 35.1 | 143 | 14.7 |
| G9P[8] | 124 | 29.7 | 78 | 26.7 | 31 | 11.8 | 233 | 24.0 |
| Uncommon | 10 | 2.4 | 11 | 3.8 | 10 | 3.8 | 31 | 3.2 |
| Mixed | 24 | 5.7 | 31 | 10.6 | 17 | 6.5 | 72 | 7.4 |
| Untypable | 5 | 1.2 | 2 | 0.7 | 14 | 5.3 | 21 | 2.2 |
| Total | 418 | 100.0 | 292 | 100.0 | 262 | 100.0 | 972 | 100.0 |
| All years | ||||||||
| Common | ||||||||
| G1P[8] | 566 | 51.3 | 460 | 50.7 | 161 | 25.4 | 1,187 | 44.9 |
| G2P[4] | 55 | 5.0 | 77 | 8.5 | 92 | 14.5 | 224 | 8.5 |
| G3P[8] | 31 | 2.8 | 28 | 3.1 | 23 | 3.6 | 82 | 3.1 |
| G4P[8] | 64 | 5.8 | 64 | 7.1 | 143 | 22.6 | 271 | 10.2 |
| G9P[8] | 292 | 26.4 | 178 | 19.6 | 122 | 19.2 | 592 | 22.4 |
| Uncommon | 19 | 1.7 | 17 | 1.9 | 16 | 2.5 | 52 | 2.0 |
| Mixed | 72 | 6.5 | 81 | 8.9 | 47 | 7.4 | 200 | 7.6 |
| Untypable | 5 | 0.5 | 2 | 0.2 | 30 | 4.7 | 37 | 1.4 |
| Total | 1,104 | 100.0 | 907 | 100.0 | 634 | 100.0 | 2,645 | 100.0 |
Temporal Distribution of Rotavirus Diarrhea Cases
The monthly distribution of 2,377 rotavirus patients with available date of onset is reported in Figure 1, according to Northern, Central, and Southern groups of Regions. Monthly data for the 3 study years were cumulated. Overall, most cases of rotavirus disease (85.0%) clustered between December–May, with minor variation between years (not shown). In Southern Italy, cases peaked in March–April, and in North-Central Regions the rotavirus peak was apparently broader starting in January with a tail in April (Fig. 1). Although infrequent in the summer months, rotavirus admissions were recorded throughout the year.
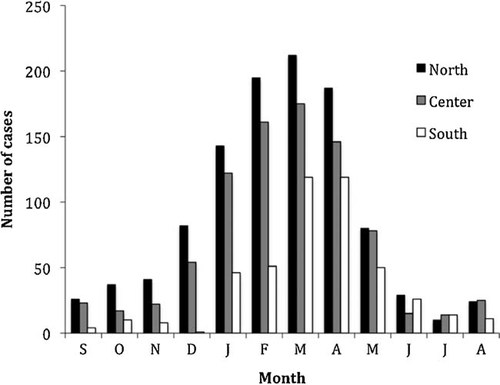
Distribution of rotavirus acute gastroenteritis cases in the North, Center, and South of Italy, by month of admission. Regions enclosed in groups are indicated. Date of onset of rotavirus diarrhea was available for a 2,377 subjects. Columns indicate the sum of cases for all 3 years of investigation.
DISCUSSION
The Rota-Net Italy Study Group is the Italian component of the 16 country-wide EuroRotaNet [Iturriza-Gomara et al., 2009], and besides providing rotavirus genotyping data from pediatric gastroenteritis cases into the European dataset, it was established to further analyze the diversity of co-circulating rotavirus strains in Italy at a Regional level. An overview of EuroRotaNet development and its results has been described elsewhere [Iturriza-Gomara et al., 2009, 2010] indicating the increasing impact of this initiative. A parallel advancement was experienced in the interest and efficiency of Regional diagnostic partners in Italy, where the number of Regions involved expanded from 7 in 2006/2007 to 13 out of total 20 forming Italy, since 2009. The number of strains genotyped by year has increased from 451 during the first season to 1253 and 993 in 2008 and 2009, respectively.
Some variation in the number of rotavirus diarrhea cases analyzed was observed between years and Regions, due to changes in resources allocated locally in different years according to regional disease prioritization plans and in response to public health emergencies. This was probably the case for almost all collaborating partners in 2009, when the H1N1 influenza pandemic threat arose and attracted most epidemiological attention, funds for laboratory diagnostics and hospital admissions. The number of 701 samples per year recommended in the EuroRotaNet sampling scheme was not reached in 2006–2007, due to the fewer regions enrolled during the starting year of the study. Nonetheless, as many as 2,697 rotavirus cases in 3 consecutive years were collected, beyond the total sample size (2,103) designed for early detection of emerging rotavirus genotypes with a prevalence of less than 1% in Italy [Iturriza-Gomara et al., 2009].
Since the total number of acute gastroenteritis cases tested in Regional Hospitals is undefined, this study cannot supply prevalence data on rotavirus in childhood. However, other specific studies conducted in limited areas of Italy confirmed rotaviruses as the major cause of severe pediatric diarrhea, as it was in the “1980s and 1990s” [Ruggeri and Declich, 1999; Medici et al., 2006; Forster et al., 2009; Giaquinto and van Damme, 2010]. Clear epidemic waves of rotavirus acute gastroenteritis were found during late winter-spring in the whole Italy, although a few patients with rotavirus infection were detected all-year round. In the South of the country, cases seem to peak with approximately one-month delay with respect to both Northern and Central Regions. Although this difference is small, it might however reflect a delayed winter in the South. This observation seems to be in loose agreement with recent findings that the rotavirus winter epidemic wave in Europe [Iturriza-Gomara et al., 2010] starts in the South-west heading on the North-east, similar to the experience in the US [Turcios et al., 2006].
The large genotype diversity (>40 G and P combinations) observed in all European countries [Iturriza-Gomara et al., 2010] is only partially reflected in Italy, suggesting that minor rotavirus strains may circulate in individual countries only. Conversely, the present data confirmed that in Italy apparent infections with “common” genotypes (G1P[8], G2P[4], G3P[8], G4P[8], and G9P[8]) occurred at an incidence rate very similar to the whole of Europe (89.1% compared to 89.5%). The same specific rates of “common” genotypes in Italy resembled closely those found for the whole European network, being 44.9% vs. 48.4%, 8.5% vs. 10.1%, 3.1% versus 4.3%, and 10.2% versus 15.1% for G1P[8], G2P[4], G3P[8], and G4P[8] viruses, respectively. Only G9P[8] strains were considerably more prevalent in Italy, being found in 22.4% of cases compared to an average of 11.6% in Europe [Iturriza-Gomara et al., 2010]. A similarly higher circulation of G9P[8] strains (25.1%) has also been reported in the bordering France during the same period [de Rougemont et al., 2011]. Compared to the results for Italy, the French investigation showed a higher mean prevalence of infections with G1P[8], that is, 59.2%, and remarkably fewer G4P[8] cases (2.2%). However, also in the case of France, the vast majority of rotaviruses typed belonged to the common types, and no obvious emergence of human-animal reassortants or zoonotic strain was detected [de Rougemont et al., 2011].
Differences in the prevalence of circulating genotypes described among European countries [Iturriza-Gomara et al., 2010] were also observed between the different Regions of Italy, and between years. To minimize fluctuations due to the low sample size of specific Regions, data were cumulated according to Northern, Central, and Southern groups of Regions (for details of grouping of the regions, see Table I). This grouping also somehow reflects differences along a North-to-South gradient in both socio-economic (industrialized/metropolitan territory towards more rural/smaller cities) and climatic (cooler/humid towards warmer/dry) parameters. Southern Regions are also more exposed to immigration particularly from Africa and other Mediterranean countries. This might partially explain the occurrence of “untypable” rotaviruses mostly in the South (4.1–5.3%), throughout the study. Accordingly, Southern Italy showed a lower (by 5–7%) frequency of “common” genotypes, and a significant decrease in G1P[8] rotavirus prevalence throughout the study period, particularly in 2006–2007 (20.9% vs. 73.6 and 61.8% in Northern and Central Regions, respectively). This was compensated by more G2P[4] and G4P[8] strains in the South (14.5% and 22.6% vs. national means of 8.5% and 10.2%, respectively). The only peculiarity of Northern Italy was a higher circulation of G9P[8] rotavirus compared to the other Regions in 2007–2009, whereas this genotype was more frequent in the South in 2006–2007. This large variability of co-circulating rotavirus genotypes by time and space in Italy remains unexplained, as it is in other countries [Santos and Hoshino, 2005]. The possibility of a differential selective pressure of vaccines towards emergence of individual genotypes cannot be invoked at present because rotavirus vaccination has progressed very slowly in all Regions of Italy. It is possible that strains which become predominant in a particular area and year would subsequently re-emerge depending on both their residual persistence in healthy carriers, animal reservoirs or the environment and/or on the immune pressure previously established in the population. It is also possible that novel strains may be locally introduced from other areas displacing formerly predominant strains based on limited antigenic differences and/or fitness to replicate in humans. As a matter of fact, it is not clear to what extent genotype diversity truly reflects diversity in host (immune system)-pathogen relationships, and the basis of rotavirus attenuation is still controversial, although it is tempting to believe that lower replication in vivo of vaccine or animal-derived strains infecting man might be involved [Vesikari et al., 2006a; Anderson, 2008; Feng et al., 2011].
Large vaccination trials and first analyses of national campaign efficacy indicate that both commercial vaccines adopted in Europe including Italy are highly efficacious against disease caused by the major rotavirus genotypes [Ruiz-Palacios et al., 2006; Vesikari et al., 2006b, 2007, 2009; Perez-Schael et al., 2007; Phua et al., 2009; Zaman et al., 2010]. Therefore, the finding that common rotavirus genotypes represent the large majority of circulating strains in Italy suggests that mass vaccination may be expected to have a significant impact on rotavirus acute gastroenteritis also in this country, as reported elsewhere. Whether vaccine efficacy will be different in different areas of Italy as a consequence of the local genotype diversities observed in this study cannot be predicted at present.
The persistence of the diversity of genotypes observed suggests per se that the situation may change easily. Novel strains carrying uncommon G and/or P genes as well as other genomic differences may emerge, as increasing exchange of people, food and animals between continents might eventually lead to importation of strains with new antigenic and virulence characteristics and particular fitness to generate epidemic or pandemic outburst of disease. Novel rotaviruses with reassorted gene segments may arise from co-infection of human or animal hosts [Gentsch et al., 2005; Martella et al., 2010]. In fact in the present study mixed infections were found in 7.6% of rotavirus patients, that may at least partially explain the 2.0% of “uncommon” rotavirus strains detected. Half of these may be derived from reassortment of “common” G and P types [Iturriza-Gomara et al., 2001], whereas the rest of them involved gene exchange from animal rotavirus strains, particularly P[6] or G[10] as recently shown by other authors [Steyer et al., 2008, 2010; Banyai et al., 2009a,b, 2010; Mukherjee et al., 2010].
The probability and outcome of mixed infections with two different rotavirus strains may depend on both the frequency of each strain co-circulating in the population, and possible interference phenomena during dual replication in the gut [Ward et al., 1996]. To explore these events, the observed numbers and genotypes of mixed infections involving common rotaviruses in Italy during the two seasons of 2007/2008 and 2008/2009 were compared with the numbers and genotypes of detected strains likely to have arisen by natural reassortment. Different types of reassortant rotaviruses were found to be grossly proportional with the numbers of observed relevant mixed infections. However, in this assessment the geographical or temporal incidence of mixed infections or natural reassortants was not considered, and thus, although logical, the correlation of low significance (P < 0.05) found is not one which will be discovered with necessity. In this context it should be noted that Muhsen et al. [2009] recently reported data from Israel where a significant correlation between the numbers of relevant mixed rotavirus infections and of natural reassortants likely to have emerged from them has been observed.
The occurrence of 37 cases where genotyping was unsuccessful might be explained by limited virus concentration in some samples and/or minor variation in the nucleotide sequence targeted by the PCR primers. Nonetheless, it cannot be excluded that some of these viruses, otherwise confirmed as rotaviruses by amplification of the VP6 encoding gene, might carry significantly different G and/or P specificities, and represent uncommon genotypes either deriving from animals or imported from abroad. In fact, the multiplex PCR used for human rotavirus detection does not include all primers needed to cover the complete genotype spectrum of group A rotavirus affecting animals, and is optimized for human strains [Iturriza-Gomara et al., 2010]. Supplementary study will be needed to characterize these untypable rotaviruses further, possibly by designing different primers for G or P gene amplification and/or by sequence analysis of other genes [Matthijnssens et al., 2008a,b].
Complex gene evolution and reassortment is likely required before animal rotavirus strains transmitted zoonotically can adapt to efficient replication and transmission in humans [Matthijnssens et al., 2008a; Martinez-Laso et al., 2009]. This is possibly the reason why strains with an animal G or P type, such as G8, G12 or P[6] in the present study, did not appear to spread significantly following their first appearance. Rotaviruses with both G and P type of animal origin were not detected at all. For this reason, it is also hard to address the question whether humans are the host where “humanization” of animal rotavirus strains first occurs, or an intermediate animal species other than the one of origin is required for strain adaptation to humans. Some underestimation of emerging animal rotaviruses may have occurred in this study, due to either lower viral load of animal viruses compared to homologous strains during dual infection, and/or to technical issues, such as unsuitable diagnostic or genotyping primers used in normal typing routine. This is one of the reasons of the importance of international laboratory networking, such as EuroRotaNet, in facilitating implementation, optimization and sharing of state-of-the-art detection methods and construction of large virus and typing data repositories fit to identify novel rotaviruses at an early stage of expansion in the human population.
APPENDIX
“RotaNet-Italy Study Group”
Maria Grazia Pompa3, Francesca Russo4, Francesca Zanella4, Dario Cesco5, Paola Sartore6, Monica Micera7, Carla Zotti8, Elena Cacello8, Luciano Balbi8, Maria Barbi9, Sandro Binda9, Valeria Primache9, Anna Maria Iorio10, Barbara Camilloni10, Michela Basileo10, Maria Cristina Medici11, Carlo Chezzi11, Laura Abelli11, Caterina Rizzo12, Giovanni Maurizio Giammanco13, Simona De Grazia13, Maria Angela Platia13, Maria Chironna14, Anna Sallustio14, Cinzia Germinario14, Maria Luisa Tanzi15, Paola Pietrosemoli16, Marcello D'Errico17, Anna Marigliano17, Nicola Comodo18, Chiara Lorini18, Vito Martella19, Sonia Storelli20, Priscilla Cocchi21, Filippo Festini22, Marta De Rosa23, Mattia Chisari23, Annibale Raglio24, Alessandra Zanchi25, Manuela Onori26, Gaetano Danzi27, Tiziana Lazzarotto28, Mino Pedroni29, Anna Mignacca30, Filippo Ansaldi31, Pierdomenico Mammì32
3CCM, Ministry of Health, Rome; 4Servizio Sanità Pubblica e Screening Regione Veneto, Venice; 5Laboratorio di Microbiologia, Ospedale di Bassano (VI); 6Laboratorio di Microbiologia, Ospedale di Camposampiero (PD); 7UO Pediatria, Ospedale di Belluno; 8Department of Public Health & Microbiology, University of Turin; 9Department of Public Health-Microbiology-Virology, University of Milan; 10Department of Medical-Surgical Specialties & Public Health, University of Perugia; 11Section of Microbiology, Department of Pathology & Laboratory Medicine, University of Parma; 12CNESP, Istituto Superiore di Sanità, Rome; 13Department of Health Promotion Sciences “G. D'Alessandro”, University of Palermo; 14Department of Biomedical Sciences & Human Oncology, Hygiene Section, University of Bari; 15Unit of Hygiene, Department of Public Health, University of Parma; 16Azienda Ospedaliera Universitaria—Policlinico di Modena; 17Department of Biomedical Sciences, Università Politecnica delle Marche, Ancona; 18Department of Public Health, University of Florence; 19Department of Veterinary Public Health, Faculty of Veterinary Medicine, University of Bari; 20UO Pediatria e Neonatologia, PO di Bisceglie (BA); 21Meyer Children Hospital of Florence; 22Department of Sciences of Women & Children Health, University of Florence; 23Azienda Ospedaliera “BMM,” Reggio Calabria; 24Laboratory of Microbiology & Virology, Ospedali Riuniti di Bergamo; 25Clinic of Infectious Diseases, University of Siena; 26UOC di Microbiologia Ospedale Pediatrico “Bambino Gesù” IRCCS, Roma; 27Laboratory of Clinical Pathology-Microbiology, Ospedale AO Moscati, Aversa; 28Clinical Unit of Microbiology, St. Orsola Malpighi University General Hospital, Bologna; 29Presidio Ospedaliero di Manerbio AOD, Brescia; 30Laboratory of Microbiology, Ospedale Maggiore di Chieri; 31Department of Health Sciences, University of Genoa; 32Azienda Sanitaria 9, Locri.




