Development and clinical validation of multiplex TaqMan® assays for rapid diagnosis of viral gastroenteritis
Abstract
There is a need to provide rapid, sensitive, and often high throughput detection of pathogens in diagnostic virology. Viral gastroenteritis is a serious health issue often leading to hospitalization in the young, the immunocompromised and the elderly. The common causes of viral gastroenteritis include rotavirus, norovirus (genogroups I and II), astrovirus, and group F adenoviruses (serotypes 40 and 41). This article describes the work-up of two internally controlled multiplex, probe-based PCR assays and reports on the clinical validation over a 3-year period, March 2007 to February 2010. Multiplex assays were developed using a combination of TaqMan™ and minor groove binder (MGB™) hydrolysis probes. The assays were validated using a panel of 137 specimens, previously positive via a nested gel-based assay. The assays had improved sensitivity for adenovirus, rotavirus, and norovirus (97.3% vs. 86.1%, 100% vs. 87.8%, and 95.1% vs. 79.5%, respectively) and also more specific for targets adenovirus, rotavirus, and norovirus (99% vs. 95.2%, 100% vs. 93.6%, and 97.9% vs. 92.3%, respectively). For the specimens tested, both assays had equal sensitivity and specificity for astrovirus (100%). Overall the probe-based assays detected 16 more positive specimens than the nested gel-based assay. Post-introduction to the routine diagnostic service, a total of 9,846 specimens were processed with multiplex 1 and 2 (7,053 pediatric, 2,793 adult) over the 3-year study period. This clinically validated, probe-based multiplex testing algorithm allows highly sensitive and timely diagnosis of the four most prominent causes of viral gastroenteritis. J. Med. Virol. 83:1650–1656, 2011. © 2011 Wiley-Liss, Inc.
INTRODUCTION
Acute gastroenteritis in the western world is associated with a substantial health and economic burden. The economic consequences are mainly attributable to primary care, hospitalization, and cost to the individual [Rodigues et al., 2007; Lorgelly et al., 2008; Anderson, 2010; Cunliffe et al., 2010]. Rapid disease diagnosis can help maximize infection control efficiency and management of the disease, whether sporadic or outbreak. Acute gastroenteritis is caused by a number of viruses, including rotavirus, norovirus, astrovirus, and group F adenoviruses [Logan et al., 2007; Anderson, 2010; Tran et al., 2010; van Maarseveen et al., 2010]. Transmission is via the fecal–oral route and clinical manifestations range from sub-clinical infection to varying degrees of fever, diarrhea, and vomiting [Elliott, 2007; Anderson, 2010]. Noroviruses and rotaviruses affect both adult and child populations, and are a primary concern for infection control [Feeney et al., 2006; Gallimore et al., 2008; Anderson, 2010; Cunliffe et al., 2010]. These viruses have an extremely low infectious dose and are very resilient in the environment. Noroviruses are members of the Caliciviridae family of ssRNA viruses, and have been identified as the most frequent cause of acute viral gastroenteritis [Glass et al., 2009; Anderson, 2010]. Noroviruses are genetically diverse viruses belonging to five recognized genogroups (GI–GIV). At least 25 antigenic types have been identified within these genogroups. GI and GII are most commonly associated with human gastroenteritis although GIV has been associated with sporadic infection [La Rosa et al., 2008; Glass et al., 2009]. Rotaviruses are members of the Reoviridae family. Uniquely, they are dsRNA viruses, and have a segmented genome which can lend itself to frequent reassortment [Estes and Kapikian, 2007; Gray et al., 2008; Greenberg and Estes, 2009]. Seven groups have been identified thus far and are categorized as serogroups A–G. Of these, serogroups A–C are known to infect humans with the majority of infections caused by serogroup A [Estes and Kapikian, 2007; Gray et al., 2008; Greenberg and Estes, 2009]. Astrovirus and adenovirus are common causes of pediatric viral gastroenteritis [Wilhelmi et al., 2003; Anderson, 2010; Tran et al., 2010]. Astroviruses belong to the family Astroviridae, genus mamastrovirus. As with norovirus, they are non-enveloped ssRNA viruses of which eight human subtypes have been identified [Walter and Mitchell, 2000; Malasao et al., 2008; Guo et al., 2010]. Subtype 1 is most commonly associated with human disease. Human adenoviruses are dsDNA viruses comprising to date a total of 52 serotypes which are grouped into seven species A–G. Although many of the 52 serotypes can be shed in feces, only serotypes 40 and 41 belonging to species F, have been shown as causative agents of pediatric gastroenteritis [Logan et al., 2006; Banyai et al., 2009; Tran et al., 2010].
All four of the above targets have been detected in a nested, multiplex PCR system; a qualitative end-point, gel-based assay, as described by O'Neill et al. [2002]. This assay was further enhanced in 2003 to include primers specific for astrovirus. The gel-based assay involves a labor-intensive algorithm with a 24 hr turn-around time in the diagnostic setting, from sample receipt to result. To decrease turn-around time, reduce labor intensity, improve sensitivity, and to have the option of quantitative detection, this gastroenteritis PCR was worked up and validated as a real-time probe-based assay.
METHODS
Clinical Samples
Fecal samples were received at the Regional Virus Laboratory, Belfast, from both hospitalized and general practice patients with gastroenteritis. Specimens were prepared aseptically as 10% suspensions in sterile phosphate-buffered saline (PBS) and centrifuged at 4,000g for 15 min, and a total of 290 µl of the clear supernatant was used for nucleic acid extraction.
Nucleic Acid Extraction
Nucleic acids from specimens were extracted using either the manual QIAamp DNA blood mini-kit or on the QIASymphony automated DNA extraction platform, in accordance with the manufacturer's instructions (Qiagen Ltd, Crawley, West Sussex, UK). Purified nucleic acid was eluted in 110 µl of supplied AE buffer.
Internal Control
An internal control, MS2 bacteriophage (purified RNA from Roche, Mannheim, Germany, cat no: 165 948) which represents encapsidated RNA, was spiked into clinical samples prior to extraction and co-amplified in the PCR to monitor nucleic acid isolation procedure and inhibition of amplification. A total of 10 µl of a 10−5 dilution of the MS2 RNA, equating to approximately 4.09 × 102 copies/ml, was added to each sample prior to extraction. MS2 amplification results in a CT fixed ±2.5 CT in the PCR for a properly isolated and uninhibited sample. The internal control primer and probe set are detailed in Table I [Rolfe et al., 2007].
| Sequence 5′–3′ | Target | Refs. | |
|---|---|---|---|
| Multiplex 1 | |||
| Rotavirus | |||
| F primer | GGCTTTAAAAGAGAGAATTTCCG | VP7 | This article |
| R Primer | TATCAGAAAGATTAGAATTGTGGTATATTC | ||
| Probe | VIC-CGG TTA GCT CCT TTT A-NFQMGB | ||
| Norovirus GI | |||
| F primer | CGY TGG ATG CGN TTY CAT GA | ORF junction 1/2 |
Kageyama et al., 2003 |
| R primer | CTT AGA CGC CAT CAT CAT TYA C | ||
| Probe 1* | FAM-AGA TYG CGR TCY CCT GTC CA-TAMRA | ||
| Probe 2** | TAMRA-AGA TYG CGR TCY CCT GTC CA-BHQ2 | ||
| Norovirus GII | |||
| F primer | CAR GAR BCN ATG TTY AGR TGG ATG AG | ORF junction 1/2 | (13) |
| R primer | TCG ACG CCA TCT TCA TTC ACA | ||
| Probe 1 | FAM-TGG GAG GGC GAT CGC AAT CT-TAMRA | ||
| Multiplex 2 | |||
| Adenovirus | |||
| F primer | CCG ACC CAC GAT GTA ACC A | Hexon gene | This article |
| R primer | GCG GTC GAC GGG CAC GAA | ||
| Probe | VIC-ACA GGT CRC AGC GAC TGA CGC-TAMRA | ||
| Astrovirus | |||
| F primer | CCG AGT AGG ATC GAG GGT | 3′ NCR | This article |
| R primer | GCT TCT GAT TAA ATC AAT TTT AA | ||
| Probe | FAM-CTT TTC TGT CTC TGT TTA GAT-NFQMGB | ||
| Internal control | |||
| MS2 λ phage | |||
| F primer | TGG CAC TAC CCC TCT CCG TAT TCA CG | 5′ NCR |
Rolfe et al., 2007 |
| R primer | GTA CGG GCG ACC CCA CGA TGA C | ||
| Probe | CY5-CAC ATC GAT AGA TCA AGG TGC CTA CAA GC-BHQ2 | ||
- *,**Alternative primer and probe label to allow simultaneous discrimination between norovirus GI and GII—note alternative probe only used in assay validation, not in 3-year clinical validation. Emission spectrum of typical TaqMan probes: FAM 483–533 nm, VIC 523–568 nm, TAMRA 558–610 nm, Cy5 618–660 nm. (Key: NFQMGB, non-fluorescent quencher minor groove binder; BHQ2, black hole quencher; ORF, open-reading frame; NCR, non-coding region.)
Multiplex Assay Design
Two multiplex assays were designed to accommodate diagnostic testing of both adult and pediatric populations. Multiplex 1 targets rotavirus and norovirus. Multiplex 2 is specifically for use with pediatric (<16 years old) specimens only as it targets astrovirus and adenovirus. Both Multiplex 1 and Multiplex 2 assays include the MS2 primer and probe set and therefore both assays are used with filter combination FAM-VIC-CY5.
Primer and Probe Design for Multiplex Assays
All PCR assays were designed specifically as reporter-quencher hydrolysis probe assays (TaqMan® Assays). Primers and probes for astrovirus, adenovirus, and rotavirus were designed de novo to target highly conserved regions (see Table I). Primer and probes were designed using Primer Express™ software version 2.0 and DNASTAR® MegAlign sequence alignment software. The rotavirus group A primers and probe were designed within the 5′ region of the VP7 gene and is a VIC-labeled minor groove binder (MGB) probe-based assay. The astrovirus PCR targets the 3′ region of the astrovirus genome and is also a FAM-labeled MGB assay. The adenovirus primers and probe were designed via the modification of the nested inner primer set and targets the 3′ end of the hexon gene [O'Neill et al., 2002]. The adenovirus PCR is a VIC-labeled TaqMan® assay. Norovirus genogroup specific primers and probe sets were adapted from published sequences [Kageyama et al., 2003]. The norovirus genogroup specific GI and GII primer and probe sets amplify the ORF1/ORF2 junction of the norovirus genome and are both FAM-labeled TaqMan® hydrolysis probes. The MS2 internal control primer and probe sequences have been published previously [Rolfe et al., 2007]. The MS2 probe incorporates a CY5 fluorophore at the 5′ end as detailed in Table I.
Multiplex RT-PCR Assays
Optimized real-time RT-PCR assays were carried out using the Superscript III Platinum® One-Step Quantitative RT-PCR System (Invitrogen, Carlsbad, CA). Both multiplex assays were performed in 10 µl reaction volumes comprising 0.2 µl Superscript™ III Reverse Transcriptase® Taq mix, 5 µl ×2 Reaction Mix (containing 0.4 mM each dNTP), 3.5 mM MgSO4, 0.2µg/µl BSA (Invitrogen), 1.0 µM each primer and probe and NFW to a volume of 8 µl. Extracted nucleic acid was denatured by heating to 95°C for 5 min followed by immediate cooling on ice. Two microliters of denatured nucleic acid was added as template giving a final reaction volume of 10 µl. Real-time RT-PCR was performed in 384-well plates using the Roche 480 LightCycler II (Roche). Cycling conditions were as follows: 50°C for 15 min, 95°C for 5 min and 45 cycles of 95°C for 10 sec and 60°C for 60 sec.
Quality Control (QC): Specificity and Sensitivity of Monoplex and Multiplex PCR Assays
To assess specificity of the assay and discount the possibility of cross-reactivity or non-specific signal detection, a panel of 137 clinical specimens which had been tested in the nested gel-based assay were re-tested against all targets with both monoplex and multiplex real-time assays. External quality assurance specimens including a HPA Enteric Unit EQ Norovirus GI and GII Panel and a National Institute of Biological Standards and Controls (NIBSC) Rotavirus Panel were tested in both monoplex and multiplex probe-based assays.
RESULTS
RT-PCR Assay Validation Results
Single-target (monoplex) RT-PCR probe-based assays were optimized for primer, probe, and magnesium concentration and combined as two multiplex RT-PCR probe-based assays. The sensitivity of the two multiplex assays was compared to the monoplex target reactions via serial dilution of extracted RNA/DNA of the target virus(es). For norovirus GI and GII, rotavirus, astrovirus, and group F adenovirus comparable sensitivities were obtained in monoplex and multiplex assays, although CT values were marginally higher in the multiplex assays (data not shown). The performance of the multiplex assay was not influenced by co-amplification of another target.
A total of 137 specimens which were positive by the nested gel-based assay [O'Neill et al., 2002] were tested with these two optimized multiplex RT-PCR assays. The probe-based assays were more sensitive for adenovirus, rotavirus, and norovirus (97.3% vs. 86.1%, 100% vs. 87.8%, and 95.1% vs. 79.5%, respectively) and also more specific for targets adenovirus, rotavirus, and norovirus (99% vs. 95.2%, 100% vs. 93.6%, and 97.9% vs. 92.3%, respectively) (see Table II). For the specimens tested, both assays had equal sensitivity and specificity for astrovirus (100%). The specificity of the two multiplex assays was further confirmed by the absence of non-specific signal generation. Of the 39 norovirus positive specimens only 1 (3%) belonged to genogroup GI. Overall the probe-based assays detected 16 more positive specimens than the nested gel-based assay (4 adenovirus, 6 rotavirus, and 6 norovirus). Both assays picked up an equal number of dual infections.
| Probe-based multiplex | Nested gel-based assay | |||
|---|---|---|---|---|
| Sensitivity (%) | Specificity (%) | Sensitivity (%) | Specificity (%) | |
| Norovirus | 91.5 | 97.9 | 79.5 | 92.3 |
| Rotavirus | 100.0 | 100.0 | 87.8 | 93.6 |
| Adenovirus | 97.3 | 99.0 | 86.1 | 95.2 |
| Astrovirus | 100.0 | 100.0 | 100.0 | 100.0 |
External Quality Control Panel Results
Quality control results showed both monoplex and multiplex assays identified the true positives and true negatives in both the HPA EQ and NIBSC control panels with 100% sensitivity and specificity.
Clinical Validation: March 2007–February 2010 Routine Diagnostic Testing Results
In March 2007 the Multiplex 1 and Multiplex 2 assays were introduced to routine service. A retrospective look-back at the data generated has shown that over a 3-year period to March 2010 a total of 9,846 specimens were processed with Multiplex 1 (7,053 pediatric specimens, 2,793 adult specimens). The pediatric specimens alone were tested with Multiplex 2.
Mean annual results of the 3-year period (see Fig. 1A,B) showed norovirus GI and GII (not differentiated in routine testing) positivity of 11% and 35% in the pediatric and adult specimens tested, respectively. One percent of the adults tested and 17% of the pediatric patients tested were rotavirus positive. Adenovirus was positive in 6% of specimens tested, 3% of specimens tested were astrovirus positive. Negative results accounted for 63% (pediatrics) and 64% (adults) of all specimens tested. The monthly/seasonal breakdown was remarkably consistent for the four virus types over the 3 years (see Figs. 2-4). Rotavirus showed a consistent winter seasonal peak in the pediatric (Fig. 2) and adult specimens tested. Numbers began to rise in December to peak over January–February, and tailed off towards late Spring, April–May. The year 2008–2009, however, showed a marked increase in both pediatric and adult populations with numbers positive reaching 639 (25.1% positivity) in the pediatrics and 35 (4.3%) in the adult populations (positivity 14.1%, 12.8% in 2007–2008 and 2009–2010, respectively, in pediatrics; positivity 1.6% in both 2007–2008 and 2009–2010 in the adult population). In both pediatric and adult populations norovirus also showed a characteristic winter peak with numbers beginning to rise in October, peaking in January, remaining high through February and beginning to decline through March. With norovirus (Fig. 3), February 2010 showed a significant increase in positivity in the adult population with 127 positive (33.6% of the 2009–2010 positives). However the overall norovirus positivity for the adult population remained stable over the 3-year period (35.4%, 36.4%, and 38.6% for 2007–2008, 2008–2009, and 2009–2010, respectively).
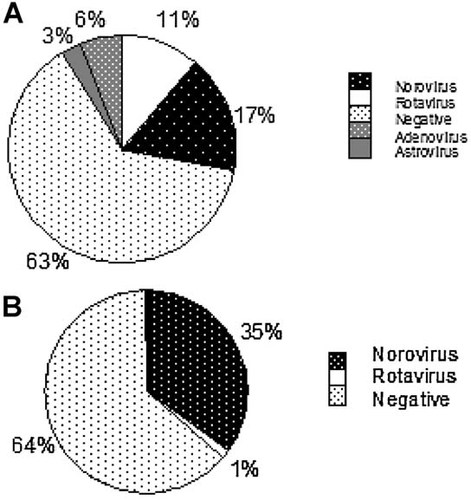
A: Average results obtained over a 3-year period, March 2007–February 2010, for 7,053 pediatric specimens processed with both multiplex assays. B: Average results obtained over a 3-year period, March 2007–February 2010, for 2,793 adult specimens processed with multiplex 1.
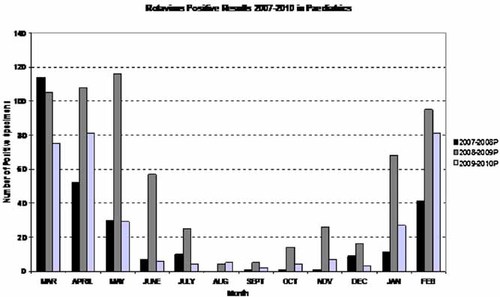
Seasonal distribution of rotavirus positive specimens in pediatrics over a 3-year period March 2007–February 2010.
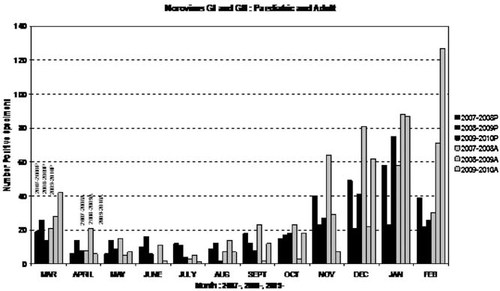
Seasonal distribution of norovirus GI/GII positive specimens in both pediatric (black) and adult (gray) specimens over a 3-year period March 2007–February 2010. P, pediatric; A, adult.
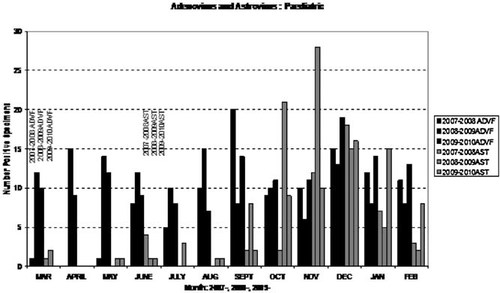
Seasonal distribution of group F adenovirus (black) and astrovirus (gray) positive specimens in the pediatric population over a 3-year period March 2007–February 2010.
Only the pediatric population was tested for group F adenovirus and astrovirus (Fig. 4). Adenovirus showed a consistent presence over the 36-month period studied, with no seasonality obvious. The average monthly positive rate for adenovirus was approximately 10/month. Astrovirus showed an autumnal/winter seasonality, with numbers beginning to rise slightly early in September, peaking in November, and tailing off towards March.
A number of mixed infections were detected, mainly a combination of rotavirus with either adenovirus, norovirus, or astrovirus, and on two occasions rotavirus with both adenovirus and norovirus (see Table III). The major occurrence of dual infections was in 2008–2009 with rotavirus and adenovirus. This was an exceptionally high year for rotavirus positivity in the pediatric population.
| Mixed infections | March 2007–February 2008 | March 2008–February 2009 | March 2009–February 2010 |
|---|---|---|---|
| Rotavirus | 4 | 6 | 12 |
| Norovirus | |||
| Rotavirus | 3 | 10 | 12 |
| Adenovirus | |||
| Rotavirus | 0 | 3 | 4 |
| Astrovirus | |||
| Norovirus | 12 | 12 | 5 |
| Adenovirus | |||
| Norovirus | 1 | 5 | 7 |
| Astrovirus | |||
| Adenovirus | 1 | 3 | 2 |
| Astrovirus | |||
| Rotavirus | 1 | 1 | 0 |
| Norovirus | |||
| Adenovirus |
The inhibition rate was seen to be 2.8% for Multiplex 1 and 3.0% for Multiplex 2. In the majority of cases inhibition was overcome via a single freeze–thaw cycle and re-extraction.
DISCUSSION
The gel-based nested multiplex assay [O'Neill et al., 2002] has been replaced by the two TaqMan™ single-round real-time multiplex assays as a regional service. Turn-around time has been reduced to same-day, or ultimately under 5 hr from specimen receipt to result issue. Approximately 3,200 fecal samples have been processed annually in these assays from 2007 to 2010 (9,846 in total). Multiplex 1 was designed specifically to target norovirus and rotavirus, viruses of particular clinical significance in gastroenteritis outbreaks where rapid result turn-around time has a direct effect on patient management and infection control [Gallimore et al., 2008; Cunliffe et al., 2010]. These viruses are also extremely important in sporadic cases of gastroenteritis, often leading to hospitalization in the very young. Multiplex 2 specifically targets group F adenovirus and astrovirus, and is only used for the pediatric population. These viral infections mostly occur in childhood and confer immunity against re-infection which accounts for the absence of these viruses in the adult population [Elliott, 2007; Anderson, 2010; Tran et al., 2010]. Another gastroenteritis virus, sapovirus, belonging to the Caliciviridae family is known to cause infections in the very young with a prevalence similar to astrovirus and will be added to the testing schedule in the near future [Svraka et al., 2010; van Maarseveen et al., 2010].
This is the first report of a probe-based diagnostic assay targeting the rotavirus VP7 gene. Due to the extreme genetic variability of rotavirus, choice of target regions is limited, and in selecting VP7 as the target gene, 100 nucleotides at the 5′ end represented the optimal choice to allow maximal detection of different isolates. Norovirus GI and GII are not distinguished routinely in our current diagnostic setting, although for the purposes of assay validation an alternative TAMRA-BHQ2 probe was used with equal sensitivity and specificity as the FAM-TAMRA probes to differentiate between the genogroups (see Table I). Rotavirus is uniquely a dsRNA virus and requires heat denaturation (95°C/5 min) prior to cDNA synthesis. All nucleic acid extracts processed via Multiplex 1 are subjected to heat denaturation, and while facilitating the detection of rotavirus, this does not alter the ability to detect the ssRNA norovirus targets. Both assays for the detection of all four targets are run simultaneously on the LC480 II using a universal RT-TaqMan PCR thermocycling program. Although the group F adenoviruses are DNA targets, this protocol does not affect sensitivity. The probe-based assays are continually monitored via in-house quality control systems and perform to an exceptionally high standard in on-going external quality control programs (HPA EQ, NIBSC).
Routine processing of the 9,846 specimens has generated valuable clinical and epidemiological data. The monthly distribution of the viruses was consistent over the 3 years, and demonstrated the seasonal incidence well, particularly with respect to norovirus and rotavirus. The norovirus outbreaks tend to establish earlier in the year than rotavirus, with January and February high months for both viruses. Winter seasonality is well established and is not reflective of enhanced testing as an outbreak protocol ensures no more than six positive specimens in any outbreak are processed. The rise in the number of positive norovirus results (n = 127) seen in February 2010 in the adult population (see Fig. 3) did not continue as the March 2010 figures (not part of the study time period) showed a drop back to n = 63. The rotavirus positives detected in the adult specimens are most likely due to re-emergence of susceptibility in the elderly population [Feeney et al., 2006].
This testing algorithm is qualitative although interpretation of real time CT values lends itself to semi-quantitation with a low CT value indicating a higher viral load, but the clinical significance of CT interpretation needs to be better established. Prolonged viral shedding, particularly with norovirus, post-acute infection was seen with a rising CT value in follow-up samples. CT values in follow-up samples can also indicate dual infection due to an overlap in infection with one virus followed by a second virus infection, rather than multiple infections at the one time.
Viral gastroenteritis in the developing world is associated with an immense disease burden and a high mortality rate. In developed countries viral gastroenteritis has low mortality rates but morbidity and economic consequences are significant. These one-step probe-based RT-PCR assays have improved turn-around time significantly, are ultra sensitive and are potentially quantitative. In the high throughput diagnostic setting this rapid, quality-assured testing enables efficient and economic service delivery.




