Hepatitis B virus genotype B with G1896A and A1762T/G1764A mutations is associated with hepatitis B related acute-on-chronic liver failure
Abstract
The existence of statistical associations between hepatitis B-related acute-on-chronic liver failure and both hepatitis B virus (HBV) genotype and mutations in the basal core promoter (BCP) and precore (PC) regions needs to be confirmed. A total of 322 patients with a chronic HBV infection, including 77 with hepatitis B-related acute-on-chronic liver failure, 109 with hepatocellular carcinoma (HCC) and 136 with chronic hepatitis B (CHB) were enrolled. The HBV genotype and the presence of mutations in the BCP/PC regions were determined by direct sequencing, and the frequencies were compared in the three patient groups. Overall, 198/322 (61.5%) were infected with genotype B and 124/322 (38.5%) with genotype C. Genotype B was significantly more frequent in patients with acute-on-chronic liver failure than CHB (92.2% vs. 60.3%, P < 0.001). As a contrast, genotype C was more common in patients with HCC than CHB (58.7% vs. 39.7%, P = 0.003). In genotype B patients, the A1762T/G1764A, A1846T, and G1896A mutations were significantly more prevalent in patients with acute-on-chronic liver failure than CHB (50.7% vs. 28.0%, P = 0.004; 59.2% vs. 34.1%, P = 0.002; 69.0% vs. 41.5%, P = 0.001, respectively). In multivariate analysis, the risk factors for acute-on-chronic liver failure were genotype B, A1762T/G1764A, and G1896A. In conclusion, CHB patients with genotype B, G1896A, and A1762T/G1764A had a higher tendency to develop liver failure than patients with genotype C. Therefore, HBV genotyping and detecting G1896A and A1762T/G1764A mutations might have important clinical implications as predictive risk factors for hepatitis B-related acute-on-chronic liver failure. J. Med. Virol. 83:1544–1550, 2011. © 2011 Wiley-Liss, Inc.
INTRODUCTION
Infection with hepatitis B virus (HBV) leads to a wide spectrum of liver disease, including the asymptomatic carrier state, fulminant hepatitis, CHB, liver cirrhosis, and hepatocellular carcinoma (HCC). Acute-on-chronic liver failure is a clinical entity with varied clinical manifestations and high mortality which results from the acute deterioration of chronic liver disease. The definition of acute-on-chronic liver failure was recently stated by the Asian Pacific Association for the Study of the Liver as an “Acute hepatic insult manifesting as jaundice and coagulopathy, complicated within 4 weeks by ascites and/or encephalopathy in a patient with previously diagnosed or undiagnosed chronic liver disease” [Sarin et al., 2009]. HBV infection is the major cause of acute-on-chronic liver failure in China.
Both viral mutations and host immune responses are believed to be involved in the pathogenesis of hepatitis B-associated acute-on-chronic liver failure. The G1896A mutation in the precore (PC) region and the A1762T/G1764A mutations in the basal core promoter (BCP) region have both been associated with the development of fulminant hepatitis [Liang et al., 1991; Omata et al., 1991; Ozasa et al., 2006; Kusakabe et al., 2009]. Other mutations including T1753V (C/A/G), T1754V (C/A/G), and G1862A in the BCP/PC regions have also been found at high frequency in fulminant hepatitis patients [Hou et al., 2002; Imamura et al., 2003]. It has been suggested that these mutations cause severe hepatitis by enhancing HBV replication or by reducing HBeAg production. However contradictory findings have been reported in a number of smaller studies from Asia and Europe, in which there were no associations between fulminant hepatitis and either the PC or BCP mutations [Liang et al., 1994; Sterneck et al., 1996; Gandhe et al., 2003; Liu et al., 2004]. With respect to viral genotype, an association between the Bj subgenotype and fulminant hepatitis has been reported in Japan [Ozasa et al., 2006; Sugauchi et al., 2006; Kusakabe et al., 2009]. There has been only one study showing associations between hepatitis B-associated acute-on-chronic liver failure and genotype B and also the T1753V, A1762T, G1764A, G1896A, and G1899A mutations [Ren et al., 2010]. Since acute-on-chronic liver failure has a high mortality and few treatment options, there is an urgent need for data that might lead to identification of viral factors that might predict the development of acute-on-chronic liver failure.
In addition, accumulating data from Asia show that genotype C is associated with a lower rate of spontaneous HBeAg seroconversion and an increased risk of HCC compared with genotype B [Kao et al., 2004; Wang et al., 2007b; Yang et al., 2008; Yu et al., 2005]. The T1753V and A1762T/G1764A mutations in the BCP region have also been reported to be associated with higher risk of HCC development, while the G1896A mutation was not associated with the risk of HCC [Yu et al., 2005; Wang et al., 2007b; Yang et al., 2008; Liu et al., 2009]. Liver failure and HCC are two distinct clinical entities characterized by excessive hepatocyte death and excessive cellular proliferation, respectively. The mechanisms by which HBV genotype and mutations in the BCP/PC regions lead to different clinical outcomes remains unclear. To address this question, it would be helpful to determine if there are differences in the HBV genotype and repertoire of BCP/PC mutations between these two clinical entities. Consequently, both clinical and virological parameters were compared in groups of patients with CHB, acute-on-chronic liver failure or HCC.
MATERIALS AND METHODS
Patients
Serum samples were obtained from 322 patients with a chronic HBV infection, including 109 patients with HCC, 77 with hepatitis B-associated acute-on-chronic liver failure and 136 with chronic hepatitis B. The 322 patients were consecutive patients in two hospitals, Nanfang Hospital and the Eighth People's Hospital of Guangzhou, and this is the first time these patients had presented for treatment for these diseases. The 109 consecutive HCC patients were admitted to the two hospitals from June 2008 to April 2010. The clinical diagnosis of HCC was confirmed by ultrasonography and/or computer tomography examination. Sera from the 77 patients with hepatitis B-associated acute-on-chronic liver failure who were admitted to the two hospitals from January 2008 to November 2009 were collected. The diagnosis of hepatitis B-associated acute-on-chronic liver failure was based on the criteria of a history of chronic hepatitis B with serum total bilirubin >10 mg/dl, prothrombin activity (PTA) <40% and recent development of complications. The 136 patients with chronic hepatitis B who presented to the two hospitals between 2008 and 2009 were included. The chronic hepatitis B was diagnosed on the basis of clinical examination and liver function tests. All patients were seropositive for the HBV surface antigen on two occasions at least 6 months apart, and seronegative for hepatitis C, D, or E viruses. Serum samples were collected on admission to or visit hospital at the first time for treatment for these diseases, and were kept at −30°C until analysis. The study was approved by the Ethics Committee of Nanfang Hospital.
Serological Assays
HBV serological markers were detected by chemiluminescent enzyme immunoassay (Abbott Laboratories, Chicago, IL, USA). Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin, and total bilirubin were determined by commercial kits. HBV DNA level was measured by real-time PCR with a detection limit of 1,000 copies/ml.
HBV Genotyping and Subgenotyping
HBV DNA was extracted from 200 µl serum using the QIAamp DNA Blood Mini Kit (QIAGEN GmbH, Hilden, Germany) according to the manufacturer's instructions. The HBV S gene was amplified for genotyping/subgenotyping with primers BS1 (5′-CCTGCTGGTGGCTCCAGTTC-3′, 56–75) and Pol2 (5′-CGGGCAACGGGGTAAAGGTTC-3′, 1,157–1,137) for the first round PCR, and if necessary, primers BS1 and P29 (5′-ATACCCAAAGACAAAAGAAAA-3′, 827–807) were used for semi-nested PCR. The PCR products were analyzed by electrophoresis on 1.5% agarose gel, stained with ethidium bromide, and sequenced with an ABI 3730 automated DNA sequencer (Applied Biosystems, Foster City, CA, USA). The sequence data were analyzed using Lasergene software suite V6.0, DNASTAR. HBV DNA sequences were aligned using CLUSTAL W software version 1.83 (DDBJ) along with HBV A-G genotype reference sequences retrieved from GenBank/EMBL/DDBJ [Wang et al., 2007a]. Genetic distances were estimated by Kimura's 2-parameter method, and phylogenetic trees were constructed by the neighbor-joining method using MEGA software V4. Bootstrap re-sampling and reconstruction with 1,000 replicates were carried out to confirm the reliability of the phylogenetic trees.
Analysis of Basal Core Promoter and Precore Mutations
The HBV BCP/PC regions were amplified by using primers P9 (5′-CATGGAGACCACCGTGAACGC-3′, 1,607–1,627) and P30A (5′-GGAGTGCGAATCCACACTCC-3′, 2,286–2,267) for the first round PCR and primers P9 and P38 (5′-CCTGAGTGCTGTATGGTGAGG-3′, 2,068–2,048) for the second round. PCR products were separated on 1.5% agarose gel and sequenced with an ABI 3730 automated DNA sequencer.
Statistical Analyses
All data were analyzed using the statistical package SPSS (version 13.0; SPSS, Inc., Chicago, IL). Chi-square, Fisher's exact, and Student's t-tests were used as appropriate. Multivariate analyses with logistic regression were used to determine the risk factors associated with acute-on-chronic liver failure development and mortality. A P-value of <0.05 was considered statistically significant.
RESULTS
Clinical and Genotypic Differences of the Patients
Table I compares the clinical characteristics of the patients. Patients with acute-on-chronic liver failure had significantly higher levels of ALT, AST, and total bilirubin, while a lower level of albumin compared with patients with chronic hepatitis B or HCC.
| Features | CHB (n = 136) | ACLF (n = 77) | HCC (n = 109) | P-value | |
|---|---|---|---|---|---|
| ACLF versus CHB | ACLF versus HCC | ||||
| Age (yrs) | 36.9 ± 11.9 | 38.2 ± 11.1 | 51.5 ± 11.4 | 0.448 | <0.001 |
| Sex (n, Male/Female) | 113/23 | 70/7 | 94/15 | 0.115 | 0.331 |
| HBeAg positive | 79 (58.1) | 22 (28.6) | 15 (13.8) | <0.001 | 0.013 |
| ALT (U/L) | 212 ± 300 | 581 ± 586 | 66 ± 57 | <0.001 | <0.001 |
| AST (U/L) | 141 ± 206 | 493 ± 704 | 75 ± 68 | <0.001 | <0.001 |
| Albumin (g/L) | 42.5 ± 6.8 | 32.2 ± 5.5 | 39.5 ± 4.7 | <0.001 | <0.001 |
| Total bilirubin (mg/dL) | 2.2 ± 3.8 | 25.0 ± 12.6 | 2.2 ± 4.1 | <0.001 | <0.001 |
- Data are given as means ± standard deviations or no. (%) of patients. CHB, chronic hepatitis B; HCC, hepatocellular carcinoma; ACLF, acute-on-chronic liver failure; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Among the 322 patients, 198 (61.5%) were infected with genotype B, all of which were subgenotype B2. One hundred and twenty-four of the 322 (38.5%) patients were infected with genotype C, of which 91were subgenotype C1 and 33 were subgenotype C2. Genotype B was detected in 92.2% (71/77), 60.3% (82/136), and 41.3% (45/109), while genotype C was detected in 7.8% (6/77), 39.7% (54/136), and 58.7% (64/109) in patients with acute-on-chronic liver failure, chronic hepatitis B and HCC, respectively. The distributions of genotype B to C between the three patient groups were significantly different (Fig. 1). Genotype B was more frequent in patients with acute-on-chronic liver failure than in patients with chronic hepatitis B (92.2% vs. 60.3%, P < 0.001). By contrast, genotype C was more common in patients with HCC than in patients with chronic hepatitis B (58.7% vs. 39.7%, P = 0.003).
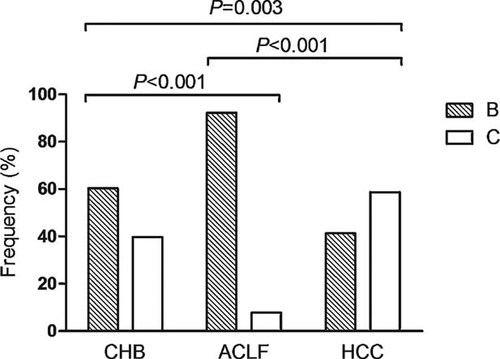
Distribution of HBV genotypes in different patient groups. The prevalence of genotype B to C in patients with chronic hepatitis B (CHB), hepatitis B-associated acute-on-chronic liver failure (HB-ACLF), and HCC were 60.3% versus 39.7%, 92.2% versus 7.8%, and 41.3% versus 58.7%, respectively. The differences were significant between CHB and HB-ACLF (P < 0.001), CHB and HCC (P = 0.003), and HB-ACLF and HCC (P < 0.001).
Frequencies of Mutations in the Core Promoter and Precore Regions Among Three Groups of Patients Who Were Infected With HBV Genotype B
Table II compares the frequency of PC/BCP mutations according to the different disease status in each HBV genotype. The A1762T/G1764A, A1846T, and G1896A were the most prevalent mutations in patients with genotype B, while T1753V and A1762T/G1764A had a high prevalence in genotype C, especially in patients with HCC. Since genotype B and C showed different mutation tendency in the BCP/PC regions, and the frequency of genotype C in liver failure patients was too low (six patients) for meaningful statistical analysis, the comparison of the frequencies of the BCP/PC mutations were only performed in patients with acute-on-chronic liver failure, chronic hepatitis B, and HCC who were infected with genotype B. Statistical analysis showed a significantly higher frequency of A1762T/G1764A, A1846T, and G1896A mutations in patients with liver failure than in patients with chronic hepatitis B (50.7% vs. 28.0%, P = 0.004; 59.2% vs. 34.1%, P = 0.002; 69.0% vs. 41.5%, P = 0.001, respectively), whereas no differences were found between patients with liver failure and with HCC. The frequencies of the T1753V, C1766T, T1768A, G1862T, and G1899A mutations were low in the patients. The prevalence of these mutations were similar among the three groups of patients except for a meaningful higher frequency of the G1899A mutation in patients with HCC than with acute-on-chronic liver failure (35.6% vs. 12.7%, P = 0.003), indicating the weak association of these mutations with acute-on-chronic liver failure.
| Viral mutations | Genotype B | Genotype C | ||||||
|---|---|---|---|---|---|---|---|---|
| CHB (n = 82) | ACLF (n = 71) | HCC (n = 45) | Pa | Pb | CHB (n = 54) | ACLF (n = 6) | HCC (n = 64) | |
| T1753V | 6 (7.3) | 2 (2.8) | 3 (6.7) | 0.286 | 0.375 | 12 (22.2) | 1 (16.7) | 44 (68.8) |
| A1762T/G1764A | 23 (28.0) | 36 (50.7) | 27 (60.0) | 0.004 | 0.327 | 31 (57.4) | 4 (66.7) | 59 (92.2) |
| C1766T | 2 (2.4) | 1 (1.4) | 4 (8.9) | 1.0 | 0.074 | 3 (5.6) | 0 (0.0) | 6 (9.4) |
| T1768A | 1 (1.2) | 1 (1.4) | 5 (11.1) | 1.0 | 0.032 | 2 (3.7) | 0 (0.0) | 9 (14.1) |
| A1846T | 28 (34.1) | 42 (59.2) | 30 (66.7) | 0.002 | 0.417 | 4 (7.4) | 2 (33.3) | 22 (34.4) |
| G1862T | 3 (3.7) | 2 (2.8) | 3 (6.7) | 1.0 | 0.375 | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| G1896A | 34 (41.5) | 49 (69.0) | 32 (71.1) | 0.001 | 0.811 | 6 (11.1) | 1 (16.7) | 8 (12.5) |
| G1899A | 7 (8.5) | 9 (12.7) | 16 (35.6) | 0.404 | 0.003 | 2 (3.7) | 2 (33.3) | 7 (10.9) |
- Data are given as no. (%) of patients. CHB, chronic hepatitis B; ACLF, acute-on-chronic liver failure; HCC, hepatocellular carcinoma. Pa, ACLF vs. CHB; Pb, ACLF vs. HCC.
Comparison of HBV DNA Level and HBeAg Negativity Among Patients With Different BCP/PC Mutation Patterns
The chronic hepatitis B patients were divided into four groups on the basis of the pattern of mutations in the BCP and PC regions for analyzing the impacts of different BCP/PC mutation on HBV DNA levels. Group 1 had no mutations in either region (BCP−/PC−), group 2 only had a BCP mutation (BCP+/PC−), group 3 only had a PC mutation (BCP−/PC+), and group 4 had mutations in both regions (BCP+/PC+). BCP+ subjects had the A1762T/G1764A double mutation, and PC+ subjects had the G1896A mutation. The HBV DNA level was significantly higher in patients with the BCP−/PC− pattern than in the other three groups (Fig. 2a). Two hundred and six patients in this study were HBeAg negative, among them 180 had either the BCP and/or the PC mutations. The frequency of HBeAg-negative patients increased in a stepwise manner in patients with the BCP−/PC− (36.1%), BCP+/PC (67.5%), BCP−/PC+ (70.0%), and BCP+/PC+ (83.3%) patterns (Fig. 2b).
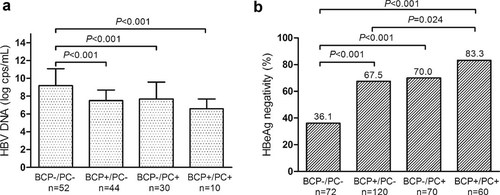
Influence of different HBV BCP/PC mutational patterns on HBV DNA levels (a) in patients CHB, and HBeAg negativity (b) in all patients. BCP+ subjects had the A1762T/G1764A double mutation, and PC+ subjects had the G1896A mutation.
Possible Factors Associated With the Development of Acute-on-chronic Liver Failure
The data from 77 patients with acute-on-chronic liver failure and 136 patients with chronic hepatitis B were used in multivariate analyses that were designed to identify significant risk factors for acute-on-chronic liver failure. HBV genotype B (OR = 4.25 [1.57–11.46], P = 0.004), the A1762T/G1764A (OR = 2.24 [1.10–4.57], P = 0.026) mutation and the G1896A (OR = 2.38 [1.12–5.08], P = 0.024) mutation were significantly associated with the development of acute-on-chronic liver failure. HBeAg negative sera, age, gender, and the T1753V, A1846T, and G1899A mutations were not risk factors for acute-on-chronic liver failure (Table III).
| Factors | Odds ratio | 95% confidence interval | P-value |
|---|---|---|---|
| Age | |||
| <40 years | 1 | ||
| ≥40 years | 0.66 | 0.31–1.51 | 0.284 |
| Sex | |||
| Female | 1 | ||
| Male | 1.68 | 0.58–4.92 | 0.34 |
| HBeAg | |||
| Positive | 1 | ||
| Negative | 2.01 | 0.91–4.44 | 0.083 |
| Genotype | |||
| C | 1 | ||
| B | 4.25 | 1.57–11.46 | 0.004 |
| T1753V mutation | |||
| Absent | 1 | ||
| Present | 0.29 | 0.07–1.25 | 0.098 |
| A1762T/G1764A mutation | |||
| Absent | 1 | ||
| Present | 2.24 | 1.10–4.57 | 0.026 |
| A1846T mutation | |||
| Absent | 1 | ||
| Present | 1.74 | 0.81–3.71 | 0.154 |
| G1896A mutation | |||
| Absent | 1 | ||
| Present | 2.38 | 1.12–5.08 | 0.024 |
| G1899 mutation | |||
| Absent | 1 | ||
| Present | 1.49 | 0.47–4.76 | 0.503 |
Risk Factors Associated With Mortality of Patients With Acute-on-chronic Liver Failure
Thirty-eight of the 77 acute-on-chronic liver failure patients survived for at least 3 months after the onset of liver failure and 39 of the 77 patients had a fatal outcome. Age, gender, serum HBeAg status, ALT, total bilirubin, PTA, HBV DNA, HBV genotype, and the A1762T/G1764A and G1896A mutations were evaluated for an association with acute-on-chronic liver failure mortality by multivariate analysis. The significant risk factors were a total bilirubin greater than 25 mg/dl (OR = 6.77 [1.12–41.03], P = 0.038), and a PTA less than 27 (OR = 14.27 [2.96–68.85], P = 0.001) (Table IV).
| Factors | Odds ratio | 95% confidence interval | P-value |
|---|---|---|---|
| Age | |||
| <40 years | 1 | ||
| ≥40 years | 0.93 | 0.21–4.20 | 0.936 |
| Sex | |||
| Female | 1 | ||
| Male | 0.48 | 0.03–7.77 | 0.608 |
| HBeAg | |||
| positive | 1 | ||
| negative | 3.12 | 0.499–19.48 | 0.224 |
| Total bilirubin | |||
| <25 mg/dL | 1 | ||
| ≥25 mg/dL | 6.77 | 1.12–41.03 | 0.038 |
| PTA (%) | |||
| ≥27 | 1 | ||
| <27 | 14.273 | 2.96–68.85 | 0.001 |
| ALT (U/L) | |||
| <580 | 1 | ||
| ≥580 | 2.61 | 0.37–18.57 | 0.338 |
| HBV DNA | |||
| <106 copies/mL | 1 | ||
| ≥106 copies/mL | 2.02 | 0.44–9.30 | 0.369 |
| Genotype | |||
| C | 1 | ||
| B | 0.22 | 0.02–2.73 | 0.237 |
| A1762T/G1764A mutation | |||
| Absent | 1 | ||
| Present | 0.85 | 0.20–3.80 | 0.836 |
| G1896A mutation | |||
| Absent | 1 | ||
| Present | 1.11 | 0.18–6.91 | 0.914 |
DISCUSSION
In the present study, by comparing the distribution of HBV genotypes in chronic hepatitis B, acute-on-chronic liver failure and HCC patients, further evidence is provided that acute-on-chronic liver failure is associated with genotype B, and HCC is associated with genotype C. Since genotype C is associated with a higher frequency of A1762T/G1764A and T1753V mutations and a lower frequency of the G1896A and A1846T mutation than genotype B, it was necessary to compare these mutation frequencies in the acute-on-chronic liver failure and chronic hepatitis B groups independent of the effect of genotype. Both the A1762T/G1764A and G1896A mutations were significantly more frequent in patients with acute-on-chronic liver failure than in patients with chronic hepatitis B. Although this finding is consistent with results observed in acute fulminant hepatitis [Ozasa et al., 2006], it is not completely in agreement with the study of acute-on-chronic liver failure performed by Ren et al. [2010]. They found similar frequencies of G1896A in acute-on-chronic liver failure patients and controls with genotype B, whereas a significantly higher prevalence of both A1762T/G1764A and G1896A mutations in acute-on-chronic liver failure patients with genotype C. They also found higher frequencies of the T1753V and G1899A mutations in their acute-on-chronic liver failure patients with genotype C. The present study indicates that T1753V and G1899A are more prevalent in HCC than in acute-on-chronic liver failure patients, suggesting an association with HCC development. Interestingly, the A1846T mutation was more prevalent in patients with acute-on-chronic liver failure and HCC than in patients with chronic hepatitis B, but the clinical significance remains unclear. In multivariate analyses, HBV genotype B, A1762T/G1764A, and G1896A mutations were independent risk factors for the development of acute-on-chronic liver failure.
It has been suggested that enhanced replication of HBV due to mutations in the BCP/PC regions might lead to fulminant hepatitis. In vitro studies have indicated that the A1762T/G1764A and G1896A mutations may enhance the replication of HBV, and the enhanced HBV replication may stimulate the host immune response and lead to fulminant hepatitis [Baumert et al., 1996; Ozasa et al., 2006]. However, HBV DNA levels were significantly lower in patients with A1762T/G1764A and/or G1896A mutations than in patients without these mutations (Fig. 2a), suggesting that immune clearance may reduce the viral load in those patients harboring A1762T/G1764A and G1896A mutations. Another possible explanation is the enhanced intracellular retention of core particles and the core particle-associated HBV DNA in the cells when A1762T/G1764A and G1896A mutations occur [Inoue et al., 2009].
HBeAg plays an important role in HBV transmission and persistence, although it is not required for HBV replication. Serum HBeAg may serve as an immune tolerogen, whereas cytosolic HBeAg serves as a target for the inflammatory immune response [Milich and Liang, 2003]. The A1762T/G1764A and G1896A mutations are the most common HBeAg-negative variants found in the BCP/PC regions that reduce or abolish HBeAg production. In accordance with a previous study [Chan et al., 1999], the vast majority (87.4%) of HBeAg-negative patients had A1762T/G1764A and/or G1896A mutations. The frequency of the HBeAg-negative state increased in a stepwise manner with the development of first the A1762T/G1764A, then the G1896A and then both mutations (Fig. 2b), suggesting that A1762T/G1764A and G1896A mutations play a major role in HBeAg-negative chronic HBV infection. Hepatocytes expressing both HBcAg and HBeAg may be more susceptible to CTL-mediated clearance than hepatocytes expressing only HBcAg, providing the HBeAg-negative mutant a selective advantage over wild-type HBV within the livers of patients with chronic infection during an immune response [Frelin et al., 2009]. It is possible that different HBV genotypes select different mutations to achieve this selective advantage. The A1762T/G1764A mutations are preferentially selected by genotypes A and C, whereas the G1896A mutation is preferentially selected by genotypes B and D [Li et al., 1993; Jardi et al., 2004; Wang et al., 2007b; Ren et al., 2010]. The loss of the immunoregulatory function of the HBeAg allows an increased CD8+ T cell response to the HBcAg to evolve, which might account for the increased frequency of fulminant hepatitis in acute HBV infections and acute-on-chronic liver failure in chronic HBV infections with HBeAg-negative variants [Liang et al., 1991; Omata et al., 1991; Sterneck et al., 1996; Ozasa et al., 2006; Kusakabe et al., 2009; Ren et al., 2010].
In addition, HBV genotype B or subgenotype Bj has a high frequency in patients with acute-on-chronic liver failure or fulminant hepatitis [Imamura et al., 2003; Ozasa et al., 2006; Sugauchi et al., 2006; Kusakabe et al., 2009; Ren et al., 2010], whereas genotype C has a high frequency in patients with HCC. The G1896A mutation is associated with genotype B, whereas the A1762T/G1764A mutation is strongly associated with genotype C. Patients with genotype B and the G1896A mutation have a high risk of developing liver failure, and patients with genotype C and the A1762T/G1764A mutation have a higher risk of developing HCC. However it is still uncertain whether the G1896A mutation functions alone, or has an additive effect with genotype B in inducing liver failure. Similarly, it is also uncertain whether genotype C and the A1762T/G1764A mutation have independent influences on the risk for developing HCC.
In summary, the HBV genotype and mutations in the BCP/PC regions may have varied impacts on the clinical sequelae of a chronic HBV infection. The chronic hepatitis B patients infected by a genotype B HBV with G1896A mutation have a greater risk of developing acute-on-chronic liver failure. Patients infected by a genotype C HBV have a greater risk of developing HCC. The A1762T/G1764A mutations may induce different clinical outcomes in different chronic hepatitis B patients. Some patients with these mutations may develop acute-on-chronic liver failure, and some others may develop HCC. The findings of the cross-sectional study in here need longitudinal study to verify the ability of these mutations as predictive factors for the development of acute-on-chronic liver failure and HCC. Further studies are also required to investigate the mechanisms by which the risk of acute-on-chronic liver failure is increased by both a genotype B HBV and the G1896A and A1762T/G1764A mutations.




