Case report: Detection of rotavirus RNA in the cerebrospinal fluid of a child with rotavirus gastroenteritis and meningism†
The authors hereby state and declare that they have no association, which might represent a conflict of interest.
Abstract
Although case reports have described detection of rotavirus (RV) in extraintestinal sites such as the liver, kidney, and central nervous system (CNS) of children with RV gastroenteritis, CNS localization in RV infection seems to be rare. RT-PCR and nucleotide sequencing detected a G1P[8] strain in the stool and cerebrospinal fluid (CSF) samples of a patient with concurrent RV-associated enteritis and CNS signs. Upon sequence analysis, the viruses detected in the CSF was identical to the virus detected in the stools. In the VP7- and VP4-based phylogenetic dendograms the strain clustered within the G1-Ic sub-lineage and the P[8]-III lineage. This study supports the hypothesis that RV infection was able to spread from the intestinal tract to the CNS, and likely played a role in the onset of neurological disease. J. Med. Virol. 83:1637–1640, 2011. © 2011 Wiley-Liss, Inc.
Rotavirus (RV) infection is localized primarily in the small intestine. Although extra-intestinal localization of RV has been reported repeatedly [Gilger et al., 1992; Makino et al., 1996; Pang et al., 1996; Hongou et al., 1998; Goldwater et al., 2001; Lynch et al., 2001; Nigrovic et al., 2002; Iturriza-Gómara et al., 2002a; Kehle et al., 2003; Nakagomi and Nakagomi, 2005; Dickey et al., 2009; Liu et al., 2009] in children with RV gastroenteritis, localization in the central nervous system (CNS) during RV infection appears to be rather rare. Also, in most cases, sequence information was not obtained on the RVs detected in the cerebrospinal fluid (CSF) of patients with CNS disease.
A 27-month-old girl, with no recent history of diseases, was admitted in January 2008 to the Paediatric Emergency Department of the University Hospital of Parma, Italy. Three days before hospital admission, the child developed vomiting, diarrhea, and fever (temperature, 37.5°C). One day before admission, the patient started displaying somnolence and rolling her eyes back. On admission, the patient was depressed, anorexic, and apyrexial. Diarrhea was still present. Also, neurological signs, including stiff neck, photophobia, and vertical nystagmus, were observed. Acute meningoencephalitis was suspected based on the clinical symptoms. At physical examination the heart rate was 150/min and oxygen saturation was 99%. No alteration was observed by cranial computed axial tomography. Initial laboratory investigations were as follows: low hemoglobin level (11.7 g/dl) and hematocrit (33.8%); leukocyte count 4.68 × 103 cells/µl (37% neutrophilis, 50.6% lymphocytes, 11.8% monocytes, 0.4% eosinophilis, and 0.2% basophilis); platelet count 244 × 103 cells/µl; high C-reactive protein level (39.1 mg/L); low urea (7 mg/dl); low creatinine (0.3 mg/dl); high aspartate aminotransferase (92 U/L); high lactate dehydrogenase (505 U/L); with sodium, potassium, calcium, and glucose unremarkable.
Intravenous rehydration, treatment with the antibiotic ceftryaxone, and the antiviral acyclovir drug, were administered. Diarrhea associated with abdominal pain persisted for 2 days after admission. On the 4th day after admission, the patient had an episode of transient epistaxis. Lumbar puncture was performed on the 1st day after admission and the CSF was collected and examined. The CSF was clear, slightly raised pressure, and the composition was as follows: protein 19 mg/dl; glucose 61 mg/dl; chlorides 122 mmol/L, 1 leukocyte/mm3, and some red blood cells. Over the subsequent days, the patient's condition gradually improved, with the stiff neck and vertical nystagmus disappeared. Intravenous therapy was stopped and the patient started eating.
Bacteriological investigations and microscopy of the CSF did not reveal any abnormalities. The CFS was negative by PCR/RT-PCR for varicella-zoster virus (VZV), herpes simplex virus 1–2 (HSV 1–2), parvovirus B19 (B19), cytomegalovirus (CMV), human herpes virus 6, adenovirus, and enterovirus (EV), using commercial assays (Nanogen Advanced Diagnostics, Turin, Italy).
Neither bacteria nor respiratory viruses were detected by routine diagnostic laboratory methods from a throat swab. In addition, HSV 1–2, B19, VZV, CMV, and EV were not detected by PCRs/RT-PCRs from the peripheral blood, obtained on the 3rd day after admission.
Rotavirus-like particles were detected by electron microscopy (EM) in the stools collected on the 3rd day after admission, and a group A RV strain, with long electropherotype (e-type), was identified by polyacrylamide gel electrophoresis [Medici et al., 2004] while cell culture and nRT-PCR for norovirus (Nanogen Advanced Diagnostics) were negative.
On the 6th day after admission, the patient was discharged with a diagnosis of meningism and RV gastroenteritis.
Rotavirus RNA was detected using a set of primers amplifying a fragment (380 bp) of the group A RV inner capsid protein VP6 gene [Iturriza-Gómara et al., 2002b] in a one-step RT-PCR (“Superscript One-Step Platinum Taq III,” Invitrogen, Foster City, CA), from the CSF and stools, but not from the plasma. Temperature conditions were as follows: 50°C for 60 min, 94°C for 2 min, 35 cycles at 94°C for 1 min, 50°C for 1 min, 68°C for 1 min, and a final extension step of 68°C for 10 min. In order to confirm these unusual findings, the analysis was repeated twice. Also, any possible contamination was ruled out during RNA extraction and RT-PCR as strict procedures (i.e., positive and negative controls and separate extraction of the samples) were adopted.
Gel-purified PCR products (Qiaquick Gel Extraction Kit, Qiagen, Hilden, Germany) were sequenced with automated sequencer 3730 DNA Analyzer (Applied Biosystems, Foster City, CA). Sequence data were analyzed using Bioedit software package and phylogenetic analysis was carried out using MEGA version 4.1 [Tamura et al., 2007]. The partial VP6 sequence (nucleotide 759–1112) generated from the CSF and the stools were 100% identical to each other. In the attempt to characterize the VP7/VP4 genotype specificity of the RV strain, the RNAs extracted from the stools and CSF were tested using several sets of primers able to predict the VP7 and VP4 specificity, as described previously [Medici et al., 2007]. In second-round PCRs, genotype P[8]-specific bands were amplified from both the CSF and the stools, while a genotype G1-specific band was obtained only from the patient's stools. In the first-round PCRs, specific amplicons of the full-length VP7 gene and the VP8* portion of the VP4 gene were generated only from the fecal sample, likely due to the higher viral load present in the feces. By sequence analysis of the VP4 and VP7 first-round amplicons obtained from the stools, the strain (PR267/08/M) was confirmed to have G1P[8] specificities. The VP4, VP6, and VP7 sequences of strain PR267/08/M have been registered in GenBank under accession numbers HQ694988, HQ694989, and HQ694990, respectively.
In the VP7 (nt 130–995) and VP4 (nt 85–1011)-based phylogenetic dendrograms (Fig. 1), strain PR267/08/M clustered within the G1 genetic lineage I, sub-lineage Ic (Fig. 1A), and the P[8] genetic lineage III (Fig. 1B).
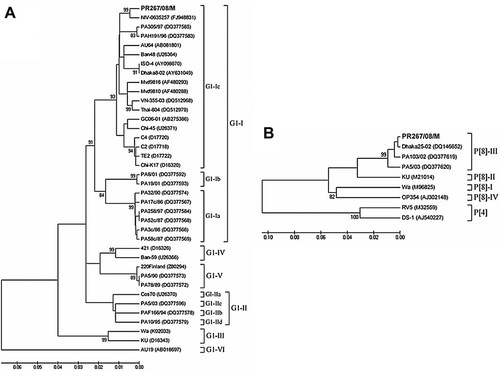
Neighbor-joining phylogenetic trees (Kimura-2-parameter model) based on partial nucleotide sequences of VP7 (nt 130–995) (A) and VP4 (nt 85–1,011) (B) genes of the rotavirus strain PR267/08/M (in boldface) obtained from the stool of a child hospitalized with gastroenteritis and meningism at Parma, Italy. The trees illustrate the genetic relationships of the PR267/08/M strain with reference strains and other established human strains available in GenBank (accession numbers are shown in parenthesis). The numbers adjacent to the nodes represent the percentage of bootstrap support (of 1,000 replicates). Bootstrap values lower than 80% are not shown.
The tropism of RVs does not seem to be absolutely restricted to enterocytes [Conner and Ramig, 1996], as RVs can infect neuronal cells in vitro [Weclewicz et al., 1998] and in vivo [Shaw et al., 1989]. Altered electrolyte balance due to dehydration or inappropriate rehydration could account for the neurological sequelae observed in some reports. However, the detection of RV RNA in CSF, as observed in this study, suggests more direct and unknown RV-mediated mechanisms able to damage the CNS and trigger the neurological signs.
In this study, common viral and bacterial infections of the CNS were excluded and only RV RNA was detected in the CSF and stools of the patient. A possible contamination of the CSF sample due to the overlying skin having come into contact with fecal material is a possibility even if it is unlikely because the CSF was obtained under sterile conditions. The RV strain detected in the CSF and in the stools were found to be identical by molecular characterization and sequence analysis, thus suggesting that the RV strain was able to spread from the intestinal tract to the CNS. As RV RNA was not detected in the patient's plasma on the 3rd day after admission, it is difficult to understand how the virus was able to reach the CNS. A possible explanation is that viremia occurred very early and only for a limited time or at low levels, below the limit of detection of the molecular assays. Indeed, it is not possible to correlate virus load in the stools with the virus load in the serum [Ray et al., 2006].
It is unclear whether the detection of RV RNA in the CSF of the patient was a serendipitous event or the neurological signs were actually caused by RV localization in the CNS. Also, in this study, some aspects of the investigations were difficult to interpret. For instance, it was not possible to amplify the VP7 gene from the CSF. This was likely due to the low viral load, below the RT-PCR detection limit, as observed by Ushijima et al. [1994]. Similar technical constraints have been reported during genotyping of RV strains [Lynch et al., 2001; Nigrovic et al., 2002] in RV-positive CSF samples.
The RV strain detected in the patient (PR267/08/M) was a common G1P[8] genotype. Other studies reviewing the role of RV in cases of gastroenteritis associated with CNS symptoms [Dickey et al., 2009; Liu et al., 2009] have observed that G1 was the most commonly identified G type [Dickey et al., 2009]. As G1P[8] RVs are largely predominant worldwide [Gentsch et al., 2005], this was not unexpected.
In the VP7 strain PR267/08/M clustered in the phylogenetic sub-lineage G1-Ic. This lineage contains an additional three strains detected in CSF samples (C2, C4, and TE2 strains) and one strain (GC06-01) detected in the stools from children with CNS disease, even if in a different sub-cluster [Ushijima et al., 1994; Shiihara et al., 2007]. This observation might be a coincidence, but is noteworthy.
Sequencing RV strains associated with neurological disease is important, since this could generate information useful for identifying genetic hallmarks of neurotropism in RV strains, or, in contrast, this information could demonstrate that no specific genetic make up or mutation is required for RVs to invade occasionally the CNS. Several genes have been suspected to be implicated in the spread of RVs from the gut to the CNS, including the NSP4, the VP3, the VP4, and the VP7. The NSP4, a non-structural protein with enterotoxic activity, is believed to interact with the enteric nervous system and this might be a route of RV dissemination [Ramig, 2004]. The VP4 and VP7, the main RV antigenic determinants, are the sites of virus–cell interactions [Kapikian et al., 2001], while the NSP3 has been suggested as the genetic determinant for RV extra-intestinal spread in the mouse model [Mossel and Ramig, 2002].
In conclusion, the increasing number of reports on CNS disease in association with RV gastroenteritis, along with the present report, suggests that RV should be included in the diagnostic algorithms of CNS disease.




