Antiviral combination therapy with peginterferon and ribavirin does not induce a therapeutically resistant mutation in the HCV core region regardless of genetic polymorphism near the IL28B gene
Abstract
An association has been reported between genetic polymorphism near IL28B gene and the prevalence of mutation of hepatitis C virus (HCV) core region residue 70, both of which have been associated with a lack of virologic response to antiviral combination therapy with peginterferon (PEG-IFN) and ribavirin. This study investigated whether PEG-IFN/ribavirin combination therapy induces amino acid (AA) mutation at residue 70 of HCV and whether genetic polymorphism near IL28B gene affects it. AA substitutions at residue 70 of the HCV core region were measured and compared before and after combination therapy in 65 non-responders and 88 relapsers to the combination therapy. In three patients in whom both wild-type AA (arginine) and mutant-type AA (glutamine or histidine) were detected at residue 70 before treatment, only mutant-type AA was identified after treatment. In two patients who had wild-type AA solely before treatment, both wild-type and mutant-type AAs were identified at residue 70 after treatment. In five patients, in whom the AA had changed at residue 70 between before and after treatment, four patients carried the TT genotype at a polymorphic locus (rs8099917) near the IL28B gene and one carried the TG/GG genotype. No difference was found in the prevalence of this change of AA at residue 70 between the TT and the TG/GG genotype. Antiviral combination therapy with PEG-IFN and ribavirin does not appear to induce mutation of HCV core region residue 70 regardless of genetic polymorphism near the IL28B gene in Japanese patients infected with HCV genotype 1b. J. Med. Virol. 83:1559–1564, 2011. © 2011 Wiley-Liss, Inc.
INTRODUCTION
Hepatitis C virus (HCV) causes chronic infection that can result in chronic hepatitis, cirrhosis of the liver, and hepatocellular carcinoma [Niederau et al., 1998; Kenny-Walsh, 1999]. The current standard therapy for patients with chronic HCV infection is the combination therapy with peginterferon (PEG-IFN) and ribavirin [Ghany et al., 2009]. Although the current treatment regimen has markedly increased the rate of patients with sustained virologic response, which indicates the eradication of HCV, only approximately 50% of patients infected with HCV genotype 1 achieve a sustained virologic response.
Many studies have investigated the potential baseline host- or virus-related factors that are associated with the lack of virologic response to IFN-based antiviral therapy. As a host-related factor, recent studies reported that genetic polymorphisms near the IL28B gene (rs8099917, rs12979860) on chromosome 19 are strongly associated with a resistance to the combination therapy in patients infected with HCV genotype 1 [Ge et al., 2009; Suppiah et al., 2009; Tanaka et al., 2009; McCarthy et al., 2010; Rauch et al., 2010]. Patients having the TT genotype at a polymorphic locus (rs8099917) near the IL28B gene show a favorable response to the combination therapy with PEG-IFN and ribavirin, whereas patients having the GG genotype or those who are TG heterozygote show a resistance to the therapy. As for virus-related factors, amino acid (AA) mutations at residue 70 in the HCV core region have been reported to be associated strongly with a resistance to PEG-IFN/ribavirin combination therapy in patients infected with HCV genotype 1b [Akuta et al., 2005, 2007a; Donlin et al., 2007]. Patients with the mutant-type AA (glutamine or histidine) at residue 70 in the HCV core region show a resistance to the combination therapy in comparison to those with the wild-type AA (arginine) at this residue. These host- and virus-related factors are both associated with the outcome of the combination therapy with PEG-IFN and ribavirin independently in a previous report [Hayes et al., 2011].
A previous study reported that the percentage of patients with the mutant-type AA at residue 70 of the HCV core region increases with the progression of chronic hepatitis, suggesting that the mutation of AA at residue 70 (from arginine to glutamine or histidine) occurs in the natural course of chronic HCV infection [Kobayashi et al., 2010a]. Several recent studies have reported a higher prevalence of the mutant-type AA at residue 70 in patients who have the TG/GG genotype of genetic polymorphism of rs8099917 near the IL28B gene, which is associated with an unfavorable response to the combination therapy with PEG-IFN and ribavirin, than in patients who have the TT genotype [Abe et al., 2010; Kobayashi et al., 2010b]. These reports suggest that the mutation of AA residue 70 of the HCV core region may occur more frequently in patients with the TG/GG genotype. Especially, the induction of this mutation may occur easily in patients who underwent PEG-IFN/ribavirin combination therapy and failed to clear HCV (non-sustained virologic response), wherein HCV obtained a resistance to combination therapy.
Mutation at HCV core region residue 70 has reportedly been associated with a hepatocarcinogenesis and an insulin resistance [Akuta et al., 2007b, 2009; Nakamoto et al., 2010]. In addition, a recent study reported that patients who have both the TG/GG genotype of rs8099917 near the IL28B gene and the mutant-type AA at residue 70 of the HCV core region have shown further resistance even to the triple therapy with telaprevir, PEG-IFN, and ribavirin [Akuta et al., 2010]. It is, therefore, important to clarify whether PEG-IFN/ribavirin combination therapy induces the mutation of the HCV core region residue 70 in patients who failed to eradicate HCV, and whether genetic polymorphism near the IL28B gene are correlated with this mutation. If so, some patients should not undergo the current standard combination therapy in order to prevent the acquisition of the resistance (i.e., mutation at residue 70).
The present study investigated the effects of the combination therapy with PEG-IFN and ribavirin and genetic polymorphisms near the IL28B gene on the mutation of HCV core region residue 70 in patients who failed to achieve a sustained virologic reponse.
PATIENTS AND METHODS
Patients and Treatment
Three hundred and forty six patients with chronic hepatitis C who had been infected with HCV genotype 1b (as assessed by amplification of core-gene sequences with polymerase chain reaction (PCR) using genotype-specific primers [Ohno et al., 1997]) and pre-treatment HCV-RNA level of >100 × 103 IU/ml [as assessed by a quantitative PCR assay (Amplicor GT-HCV Monitor, Version 2.0; Roche Molecular Systems, Pleasanton, CA)] underwent antiviral combination therapy with PEG-IFN and ribavirin between January, 2007 and December, 2009 at the Ogaki Municipal Hospital or the Nagoya University Hospital. Of these patients, 19 patients dropped out and their outcome could not be defined. Among the remaining 327 patients, 274 patients who gave written informed consent for genetic analyses were enrolled to the study (Fig. 1). No patients were coinfected with hepatitis B virus or human immunodeficiency virus.
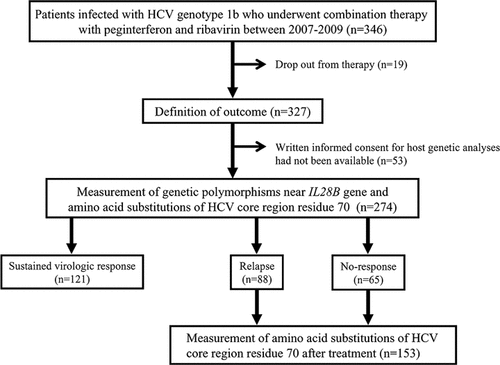
Schematic representation of the study design.
All patients were given PEG-IFN alpha-2b (Pegintron, Schering-Plough, Tokyo, Japan) weekly and ribavirin (Rebetol, Schering-Plough) daily. The initial doses of PEG-IFN and ribavirin and the dose reductions were according to the manufacturer's recommendations. All patients were scheduled to undergo 48 weeks of the treatment. Some patients had an extended treatment duration of up to 72 weeks. In some patients, the treatment was discontinued before 48 weeks because they had a low likelihood of achieving a sustained virologic response, when serum HCV-RNA was positive 24 weeks after starting the therapy. The outcomes of the combination therapy were classified as a sustained virologic response when serum HCV-RNA became undetectable during the treatment and remained undetectable for 6 months after the treatment ended (i.e., eradication of HCV), a relapse when the serum HCV-RNA became undetectable during the treatment period but returned detectable after the treatment, and no-response when the serum HCV-RNA remained detectable during and after the treatment period.
The study protocol was in compliance with the Helsinki Declaration and was approved by the ethics committee of the Ogaki Municipal Hospital and the Nagoya University School of Medicine. Written informed consent was obtained from all patients prior to the study for the measurement of genetic polymorphism of rs8099917 near IL28B gene and AA substitution of HCV core region residue 70, and for the use of the laboratory data.
Measurements of Genetic Polymorphism Near the IL28B Gene and Amino Acid Substitution of the HCV Core Region Residue 70
Genotyping of polymorphisms of the rs8099917 locus near the IL28B gene was carried out in all 274 patients using a Taqman SNP assay (Applied Biosystems, Foster City, CA) according to the manufacturer's guidelines. A pre-designed and functionally tested probe was used for rs8099917 (C__11710096_10, Applied Biosystems).
The AA at residue 70 of the core region of HCV was measured before the treatment in all patients. In patients who failed to achieve a sustained virologic response, that is, patients who showed a relapse or no-response, the AA identity was measured at residue 70 after the treatment and compared pre- to post-treatment AA identity at this residue (Fig. 1). The AA at residue 70 after the treatment was measured in serum samples obtained at the end of treatment in patients who showed no-response. In patients with a relapse, it was measured in serum samples obtained upon the reappearance of HCV-RNA after the completion of the therapy. The AA identity was analyzed by direct nucleotide sequencing according to a previous report [Akuta et al., 2007c]. The primer pairs used for PCR for direct sequencing the HCV core region were 5′-GCCATAGTGGTCTGCGGAAC-3′ (outer, sense primer), 5′-GGAGCAGTCCTTCGTGACATG-3′ (outer, antisense primer), 5′-GCTAGCCGAGTAGTGTT-3′ (inner, sense primer), and 5′-GGAGCAGTCCTTCGTGACATG-3′ (inner, antisense primer).
Statistical Analysis
The chi-square test was used to analyze the differences in percentages between groups.
RESULTS
Patient Characteristics and the Outcome of the Combination Therapy
The characteristics of study patients are shown in Table I. The study patients comprised 139 males (50.7%) and 135 females (49.3%), with a mean age of 58.0 ± 10.4 years. The grade of liver fibrosis according to the METAVIR score [The French METAVIR Cooperative Study Group, 1994] was F0 in 31 patients (11.6%), F1 in 122 patients (45.9%), F2 in 75 patients (28.2%), and F3 in 38 patients (14.3%). Analysis of the genetic polymorphism of the rs8099917 near the IL28B gene indicated 202 patients (73.7%) had the TT genotype, three patients (1.1%) had the GG genotype, and the remaining 69 patients (25.2%) were TG heterozygous. Before the treatment, 204 patients (74.4%) carried HCV with the wild-type AA at residue 70 of the HCV core region, 64 patients (23.4%) carried the mutant-type AA at residue 70, and both the wild-type AA and the mutant-type AA were identified at residue 70 in the remaining six patients (3.5%).
| Age (years) | 55.9 ± 11.2 |
| Sex (female/male) | 135 (49.3)/139 (50.7) |
| Body weight (kg) | 58.0 ± 10.4 |
| Alanine aminotransferase (IU/L) | 64.5 ± 56.3 |
| Aspartate aminotransferase (IU/L) | 53.7 ± 42.2 |
| Gamma-glutamyl transpeptidase (IU) | 49.7 ± 48.5 |
| Alkaline phosphatase (IU/L) | 267.9 ± 100.6 |
| Albumin (g/dl) | 4.07 ± 0.38 |
| Total bilirubin (mg/dl) | 0.79 ± 0.30 |
| White blood cell count (/µl) | 4933 ± 1331 |
| Hemoglobin (g/dl) | 14.0 ± 1.4 |
| Platelet count (×103/µl) | 164 ± 50 |
| Liver histology-activity (A0/A1/A2/A3)a | 2 (0.7)/147 (55.3)/99 (37.2)/18 (6.8) |
| Liver histology-fibrosis (F0/F1/F2/F3)a | 31 (11.6)/122 (45.9)/75 (28.2)/38 (14.3) |
| HCV-RNA concentration (log10 IU/ml)b | 6.34 ± 0.54 |
| Genetic polymorphisms near the IL28B gene (TT/TG/GG)b | 202 (73.7)/69 (25.2)/6 (2.2) |
| Amino acid at HCV core 70 (wild type/mutant type/both)c | 204 (74.4)/64 (23.4)/6 (2.2) |
| Response (SVR/relapse/NR) | 121 (44.2)/88 (32.1)/65 (23.7) |
- HCV, hepatitis C virus; SVR, sustained virologic response; NR, no-response.
- Percentages are shown in parentheses.
- a Liver biopsy was not performed in eight patients.
- b rs8099917 genetic polymorphism
- c Before the treatment.
As a final outcome, 121 patients (44.2%) achieved a sustained virologic response, 88 patients (32.1%) relapsed, and the remaining 65 patients (23.7%) showed no-response (Fig. 1). Treatment was discontinued before 48 weeks in 11 of 65 patients who showed no-response because HCV-RNA remained detectable in serum 24 weeks after starting the therapy. The identity of the AA 70 of the core region of HCV was determined after the treatment in serum obtained at the discontinuation of the therapy in these 11 patients. Table II shows the association between the genetic polymorphisms of the rs8099917 near the IL28B gene, the AA substitutions of the HCV core region residue 70, and the outcome of the combination therapy. The wild-type AA was more frequently identified at residue 70 in patients with the TT genotype in comparison to those with the TG/GG genotype (82.2% vs. 52.8%, P < 0.0001). The rate of a sustained virologic response was significantly higher in patients with the TT genotype than those with the TG/GG genotype (107 of 202 patients, 53.0% vs. 14 of 72 patients, 19.4%, P < 0.0001), as well as being higher in patients carrying HCV with the wild-type AA at residue 70 of the core region than those with the mutant-type AA at this residue (101 of 204 patients, 49.5% vs. 19 of 64 patients, 29.7%, P = 0.0083, one patient had both the wild-type and the mutant-type AAs).
| Genetic polymorphism of rs8099917 near IL28B gene | Amino acid at residue 70 of the HCV core region | ||
|---|---|---|---|
| Wild type (n = 204) | Mutant type (n = 64) | Wild type + mutant type (n = 6) | |
| TT (n = 202) | 166 (92/60/14) | 31 (14/9/8) | 5 (1/2/2) |
| TG/GG (n = 72) | 38 (9/9/20) | 33 (5/7/21) | 1 (0/1/0) |
- Outcomes of the combination therapy with peginterferon and ribavirin are shown in parentheses as sustained virologic response/relapse/no-response.
Comparison of the Amino Acid at Residue 70 of the HCV Core Region Before and After the Combination Therapy in Patients Who Showed a Relapse or No-Response
Table III shows the comparison of the AA at residue 70 of the HCV core region before and after the combination therapy in patients who showed a relapse or no-response, according to the genetic polymorphisms of the rs8099917 near the IL28B gene. In three of five patients in whom both the wild-type and mutant-type AAs had been identified at residue 70 of the HCV core region before treatment, only the mutant-type AA was identified at this residue after the treatment. All three of these patients (two no-responders and one relapser) had the TT genotype of the rs8099917. Both the wild-type and mutant-type AAs were identified at residue 70 after the treatment in two no-responders in whom only the wild-type AA had been identified before the treatment. One of them had the TT genotype at the rs 8099917 and the other patient was TG heterozygous. No change in the HCV core region residue 70 was found after the treatment in patients with the mutant-type AA at this residue before the treatment.
| Amino acid at HCV core region residue 70 | |||
|---|---|---|---|
| Before treatment | After treatment | ||
| Wild type | Wild + Mutant | Mutant type | |
| (A) Genetic polymorphisms near the IL28B gene (rs8099917): TT (n = 91) | |||
| No-responders (n = 24) | |||
| Wild type (n = 14) | 13 | 1 | 0 |
| Wild + mutant (n = 2) | 0 | 0 | 2 |
| Mutant type (n = 8) | 0 | 0 | 8 |
| Relapsers (n = 71) | |||
| Wild type (n = 60) | 60 | 0 | 0 |
| Wild + mutant (n = 2) | 0 | 1 | 1 |
| Mutant type (n = 9) | 0 | 0 | 9 |
| (B) Genetic polymorphisms near the IL28B gene (rs8099917): TG/GG (n = 57) | |||
| No-responders (n = 41) | |||
| Wild type (n = 20) | 19 | 1 | 0 |
| Wild + mutant (n = 0) | 0 | 0 | 0 |
| Mutant type (n = 21) | 0 | 0 | 21 |
| Relapsers (n = 17) | |||
| Wild type (n = 9) | 9 | 0 | 0 |
| Wild + mutant (n = 1) | 0 | 1 | 0 |
| Mutant type (n = 7) | 0 | 0 | 7 |
- HCV, hepatitis C virus.
DISCUSSION
The present study investigated whether the combination therapy with PEG-IFN and ribavirin causes the mutation of residue 70 of the HCV core region, and whether the genetic polymorphisms of the rs8099917 locus near the IL28B gene influence this mutation. It is thought to be important to verify this issue, because it may be advisable to avoid the treatment of patients who have the TG/GG genotypes by the combination therapy with PEG-IFN and ribavirin so as to avoid an acquisition of the further resistance to emerging new therapies against HCV, as well as to avoid a potential enhancement of hepatocarcinogenesis.
The mutation of the AA at residue 70 was not observed before and after the treatment in all patients who had failed to achieve a sustained virologic response. The mutant-type AA was identified solely at residue 70 after the treatment in three patients who had both the wild-type and the mutant-type AAs at residue 70 before the treatment. This could be due to the selection of HCV strains with the mutant-type AA at residue 70 by the combination therapy with PEG-IFN and ribavirin, as reported previously [Kurbanov et al., 2010]. In two patients who carried only the wild-type AA before the treatment, the HCV with the mutant-type AA at residue 70 was also detected with the persistence of the wild-type AA at this residue after the treatment. The very minor HCV strain with the mutant-type AA at residue 70 which were not detected before the treatment may have been detected after the treatment due to the reduction of HCV with the wild-type AA at residue 70 by the combination therapy. Indeed, HCV with the mutant-type AA at core region residue 70 was not detectable in serum 6 months after the end of the combination therapy, suggesting that it returned to being a very minor population (data not shown). These two phenomena were observed in patients with both the TT genotype of the rs8099917, that is associated with a favorable response to the combination therapy and those with the TG/GG genotypes that is associated with an unfavorable response, without difference in the prevalence according to the genetic polymorphisms at the rs8099917 near the IL28B gene.
In conclusion, PEG-IFN/ribavirin combination therapy does not appear to induce the mutation of the AA at the HCV core region residue 70 regardless of the genetic polymorphism near the IL28B gene in Japanese patients infected with HCV genotype 1b. The combination therapy can be attempted regardless of the genetic polymorphisms near the IL28B gene in treatment-naïve patients without the anxiety for the acquisition of the further resistance to the antiviral therapy. However, future studies should be undertaken to confirm the absence of the mutation at residue 70 of the HCV core region induced by the combination therapy with PEG-IFN and ribavirin. In addition, the effect of the genetic polymorphisms near the IL28B gene on the mutation of the AA at the HCV core region residue 70 should be investigated in the long-term observation of the natural course of chronic hepatitis C.




