Aboriginal Taiwanese hepatitis B carriers have more favorable viral factors than Han Chinese carriers
Abstract
Several viral factors are associated with disease progression in hepatitis B virus (HBV) carriers. Compared with Taiwanese Han Chinese, Taiwanese aborigines have a higher prevalence of chronic HBV infection and a higher standardized mortality rate of chronic liver diseases but a lower standardized mortality rate of hepatocellular carcinoma (HCC). The aim of this study was to investigate whether aboriginal Taiwanese HBV carriers have more favorable viral factors which reduce the risk for HCC than Han Chinese carriers. Blood samples from 3,488 HBV carriers (1,527 aborigines and 1,961 Han Chinese) were assayed for aminotransferases, hepatitis B e antigen (HBeAg), HBV DNA, and HBV genotype. Aboriginal HBV carriers had a lower HBeAg-positive rate (5.3% vs. 10.2%, P < 0.0001) and a lower viral load of HBV DNA > 2,000 IU/ml (27.4% vs. 36.7%, P < 0.0001) but a higher rate of alcohol consumption (40.0% vs. 19.3%, P < 0.0001) than Han Chinese carriers. The prevalence of HBV genotype B in aboriginal carriers (92.7%) was significantly higher than that in Han Chinese carriers (72.7%) in all age groups (P < 0.05). In addition, patients with rare genotype D infections were clustered in a township in southern Taiwan. In conclusion, aboriginal Taiwanese HBV carriers have more favorable viral factors than Han Chinese carriers, which may be partly responsible for the lower standardized mortality rate of HCC in Taiwanese aborigines. J. Med. Virol. 83:1326–1331, 2011. © 2011 Wiley-Liss, Inc.
INTRODUCTION
Taiwan has a long history of immigration and is generally believed to be the place of origin of many Austronesian groups. For the purpose of this study, the researchers have divided the current population in Taiwan into two groups: the Han Chinese and the aborigines. The Han Chinese include (1) the so-called “native Taiwanese”—the southern-Min dialect speakers whose ancestors moved to Taiwan before 1949; (2) the Hakka-speaking people; and (3) the mainlanders, who moved to Taiwan after 1949. The aborigines only consist of about 2% of the total population. Thus far, 14 aboriginal tribes have been recognized by the government, and each tribe has distinct language, culture, traditions, customs, and social structure. Most of the aboriginal population live in mountainous areas in eastern and southern Taiwan (Council of Indigenous People Taiwan, 2007).
Worldwide, nearly 400 million people have chronic hepatitis B virus infection (HBV), and 15–40% suffer from cirrhosis, hepatic failure, or hepatocellular carcinoma (HCC) during their lifetime [Beasley, 1988; Lee, 1997; Kao and Chen, 2002; Sorrell et al., 2009]. Several host and viral factors increase the risk of developing HCC in HBV carriers, including male gender, old age, abnormal alanine aminotransferase (ALT) level, hepatitis B e antigen (HBeAg)-positive status, cirrhosis, persistently high levels of HBV DNA, HBV genotype C, wild-type precore sequence, and basal core promoter (BCP) mutations [Yang et al., 2008, 2010]. There are at least 8 known HBV genotypes. In Taiwan and East Asia, genotypes B and C are the most common [Kao, 2002]. In addition, HBV genotype C is associated with more severe liver disease, including cirrhosis and HCC, than genotype B [Kao, 2002], because patients with genotype C are more likely to be HBeAg-positive and have a higher serum level of HBV DNA than those with genotype B [Kao, 2003].
In Taiwan, HBV infection is the most common cause of chronic hepatitis, cirrhosis, and HCC [Chen, 1993]. Although the rate of hepatitis B surface antigen (HBsAg) carriage in vaccinated individuals is only 1.2%, the rate is still 15–20% in adults who were born before the implementation of the nationwide HBV vaccination program. However, the prevalence of chronic HBV infection varies in different parts of Taiwan [Chen et al., 2007a]. For example, people residing in the mountainous areas have a higher rate of HBsAg carriage [Lin et al., 2000; Wang et al., 2006]. In addition, Taiwanese aborigines have a higher HBV carrier rate [Chung et al., 1988] and a higher standardized mortality rate of chronic liver diseases than Han Chinese (77.6/100,000 vs. 13.7/100,000, respectively) but a slightly lower standardized mortality rate of HCC than Han Chinese (27.4/100,000 vs. 28.1/100,000, respectively; Council of Indigenous Peoples Taiwan, 2007). To understand these paradoxical epidemiological data, this study investigated whether aboriginal Taiwanese HBV carriers have more favorable viral factors which reduce the risk for HCC than Han Chinese carriers.
METHODS
Subjects
HBsAg-positive individuals who lived in rural areas of Taiwan were recruited from the Liver Disease Prevention and Treatment Research Foundation in Taiwan and local government health centres. All of the subjects agreed to participate in the study and provided written informed consent before being enrolled. In addition, the procedures for this study were approved by the Research Ethics Committee of the National Taiwan University Hospital (Protocol ID No. 200801059R) and were performed in accordance with the ethical standards of the Helsinki Declaration.
Basic Information
A questionnaire was used to collect basic demographic and clinical data from subjects, such as gender, date of birth, ethnic group, birthplace, place of residency, presence of tattoos or body piercings, and history of transfusions, needle sharing, surgical treatments, smoking, and alcohol consumption. Their body weight, body height, and waist circumference also were measured.
Blood Tests and Abdominal Ultrasound
Blood samples were collected and assayed for aspartate aminotransferase (AST) and ALT by using a serum chemistry autoanalyzer (Hitachi 7150, Tokyo, Japan), α-fetoprotein (AFP) by using a commercial enzyme-linked immunosorbent assay (ELISA, AFP EIA kit; General Biologicals Corporation, Hsin-Chu, Taiwan), and HBeAg and hepatitis B e antibody (anti-HBe) by using a commercial ELISA (EASE BN-96, General Biologicals Corporation).
In addition, fatty liver and cirrhosis were identified by examining the liver with ultrasound (Toshiba SSA-320A, Tochigi, Japan). The diagnostic criteria for cirrhosis were coarse echotexture, nodular surface, and an irregularly narrow right hepatic vein and for fatty liver were increased echogenicity of the liver parenchyma and blurring of the vascular margins.
Quantification of Serum Hepatitis B Virus DNA
Viral nucleic acid was extracted from 200 µl of serum using the Viral DNA purification kit (QIAamp DNA Mini Kit, Qiagen, Valencia, CA), and then eluted in 50 µl of sterilized water. The purified DNA was quantified by using the method of Liu et al. [2007] with some modifications. Briefly, a 67-nucleotide fragment in the S region of the HBV genome was amplified and quantified by real-time polymerase chain reaction (RT-PCR) in an ABI7900 Real-Time PCR System (Applied Biosystems, Carlsbad, CA) with the primers shown in Table I. The amount of HBV DNA was determined by probing primer (Table I) according to HBV standard (Acrometrix, Benicia, CA). The dynamic range of HBV DNA detection is 50–2 × 109 IU/ml. The results of this real-time PCR method for 20 randomly selected samples were highly correlated with those from the AMPLICOR HBV MONITOR® Test, v2.0 (Roche Diagnostics, Basel, Switzerland; R2 = 0.98–1.0).
| Primers | Sequences |
|---|---|
| Quantification of HBV DNA | |
| Forward primer | 5′-AGTGGGCCTCAGTCCGTTT-3′ |
| Reverse primer | 5′-AGCCCTACGAACCACTGGAACA-3′ |
| Probing primer | (FAM)-5′-TCCTGGCTCAGTTTACTAGTGCCA-3′-(TAMRA) |
| Nested PCR | |
| Universal primers | |
| HBV-2821F | 5′-GGTCACCATATTCTTGGGAACAA-3′ |
| HBV-2437R | 5′-CCGAGATTGAGATCTTCTGCGAC-3′ |
| Genotype B-specific primers | |
| HBV-B1470F | 5′-CCGCTTGGGGCTCTACCGCCCG-3′ |
| HBV-B1660R | 5′-CTCTTATGCAAGACCTTGGGCAGGTTCC-3′ |
| Genotype C-specific primers | |
| HBV-C3164F | 5′-CTCCCATCTCTCCACCTCTAAGAGACAGT-3′ |
| HBV-C190R | 5′-CAGGGGTCCTAGGAATCCTGATGTKG-3′ |
| Genotype D-specific primers | |
| HBV-D2843F | 5′-ACAGCAGGGGCAGAATCTTTCCACCAG-3′ |
| HBV-D2958R | 5′-GTTGGGATTGAAGTCCCAATCTGGA-3′ |
- F, forward; R, reverse; K, T or G; FAM, carboxyfluorescein; TAMRA, carboxytetramethylrhodamine.
Genotyping of Hepatitis B Virus DNA
Nested and multiplex PCR were used with appropriate primers (Table I) to determine HBV genotypes, as described previously [Naito et al., 2001; Kirschberg et al., 2004; Chen et al., 2007b]. Briefly, universal primers (HBV-2821F and HBV-2437R) were used in the first PCR to amplify the HBV genome, and then genotype-specific primers were used in the second PCR to differentiate genotypes B, C, and D. Ten microliters of amplified DNA were electrophoresed on a 2% agarose gel, stained with ethidium bromide, and visualized with ultraviolet (UV) light. Then, the HBV genotype of each sample was determined by identifying genotype-specific patterns of DNA fragments.
To confirm the accuracy of PCR-based HBV genotyping, full-length genome sequencing of some samples were performed [Gunther et al., 1995]. First, HBV genomic DNA was PCR-amplified with universal primers. Then, the PCR products were purified by the GFX™ Polymerase Chain Reaction DNA and Gel Band Purification Kit (Amersham Biosciences, GE Healthcare, Waukesha, WI). Finally, the complete genome sequence was assembled from six PCR-amplified sequences by using DNASTAR Lasergene® v8.0 SeqMan Pro software (DNASTAR, Inc., Madison, WI).
Statistical Analysis
Statistical analyses were performed by using SAS software version 9.1.3. Categorical data were analyzed by the chi-squared test, while continuous data were analyzed by Student's t-test. Logistic regression was used for prediction of the probability of occurrence of an event. A P-value <0.05 was considered statistically significant.
RESULTS
Clinical and Virological Characteristics Subjects
A total of 3,488 HBsAg-positive subjects (1,527 aborigines and 1,961 Han Chinese) were recruited from 42 rural townships in Taiwan. As shown in Table II, compared with Han Chinese subjects, aboriginal subjects had a lower ratio of males to females and were slightly older, less likely to be HBeAg-positive, and more likely to consume alcohol. In addition, aborigines had higher levels of AST and similar levels of ALT but lower levels of HBV DNA than Han Chinese subjects.
| Han Chinese (n = 1961) | Aborigines (n = 1527) | P-value | |
|---|---|---|---|
| Gender (male:female ratio) (% male) | 1,056:905 (53.9%) | 664:863 (43.5%) | <0.0001 |
| Age (mean (SD)) (years) | 50.0 (13.6) | 51.6 (13.3) | 0.0006 |
| HBeAg-positive status | 199 (10.2%) | 81 (5.3%) | <0.0001 |
| AST (mean (SD)) (U/L) | 31.6 (27.1) | 37.6 (30.6) | <0.0001 |
| ALT (mean (SD)) (U/L) | 39.4 (45.9) | 39.3 ± 32.6 | 0.9034 |
| ALT > ULN (40 U/L) | 554 (28.3%) | 477 (31.2%) | 0.0551 |
| HBV DNA (mean (SD)) (IU/ml) | 4.3 × 107 (3.1 × 108) | 2.6 × 106 (2.5 × 107) | <0.0001 |
| HBV DNA >2,000 IU/ml | 720 (36.7%) | 419 (27.4%) | <0.0001 |
| Alcohol consumptiona | 352 (19.3%) | 517 (40.0%) | <0.0001 |
- ULN, upper limit of normal.
- a No data for 374 subjects.
Ultrasound Findings
Ultrasound examinations showed that the frequency of fatty liver was significantly higher in aborigine subjects than Han Chinese subjects (55.2% vs. 50.3%, P = 0.017). However, the percentage of cirrhosis in these two groups was similar.
Distribution of Hepatitis B Virus Genotypes
HBV genotyping was performed in 1,335 (38.3%) samples with a HBV DNA level >103 IU/ml. There were six types of pure and recombinant HBV genotypes, including B (1,009 samples, 75.6%), C (236 samples, 17.7%), D (13 samples, 1.0%), B + C (66 samples, 4.9%), B + D (10 samples, 0.7%), and B + C + D (1 sample, 0.1%).
When pure genotypes B and C infections were the most common genotypes in Taiwan which were considered for analysis, the prevalence of genotype B was 80.6%. However, the prevalence increased to 89.1% after excluding the individuals who lived on offshore islands because most of their ancestors originated from mainland China and had primarily genotype C [Liu et al., 2002]. When the prevalence rate was analyzed according to geographic areas, the prevalence of genotype B was 92.7% in the eastern rural area, 91.2% in the central rural area, 90.2% in the northern rural area, and 82.0% in the southern rural area of the main island of Taiwan but only 40.3% in the offshore islands. This result indicated that offshore islanders have a higher prevalence of HBV genotype C.
Clinical and Virological Characteristics of Hepatitis B Virus Carriers With Different Genotypes
Among the 1,335 HBV samples which were genotyped, the HBeAg status was known in 1,185 samples. Compared with subjects who had HBV genotype B, those with genotype C were younger, were more likely to be HBeAg-positive, and had higher levels of serum ALT and HBV DNA (Table III).
| Genotype B (n = 955) | Genotype C (n = 230) | P-value | |
|---|---|---|---|
| Gender (male:female ratio) (% male) | 483:472 (50.6%) | 129:101 (56.1%) | 0.1332 |
| Age (mean (SD)) (years) | 50.3 (13.3) | 48.1 (12.6) | 0.0202 |
| HBeAg-positive status | 98 (10.3%) | 86 (37.4%) | <0.0001 |
| AST (mean (SD)) (U/L) | 37.5 (32.5) | 41.7 (38.1) | 0.0880 |
| ALT (mean (SD)) (U/L) | 44.2 (49.0) | 53.6 (57.2) | 0.0121 |
| ALT > ULN (40 U/L) | 341 (35.7%) | 99 (43.0%) | 0.0387 |
| HBV DNA (mean (SD)) (IU/ml) | 7.3 × 106 (7.2 × 107) | 2.5 × 108 (8.1 × 108) | <0.0001 |
| HBV DNA >2,000 IU/ml | 516 (54.0%) | 200 (87.0%) | <0.0001 |
| Alcohol consumptiona | 217 (25.4%) | 61 (28.1%) | 0.4231 |
- ULN, upper limit of normal.
- a No data for 115 subjects.
Logistic regression analysis showed that various factors, such as old age, not originally an offshore island resident, being an aborigine, lower serum levels of ALT, lower viral load of HBV DNA, or the presence of anti-HBe antibodies, were associated with HBV genotype B infection (P < 0.05). On the other hand, a higher HBV viral load and an HBeAg-positive status were associated with HBV genotype C infection (P < 0.001).
Comparison of the Prevalence of Hepatitis B Virus Genotypes in Han Chinese and Aboriginal Carriers
Table IV shows the distribution of HBV genotypes in 1,178 subjects stratified by HBeAg status and ethnic groups. Notably, aboriginal HBV carriers were more likely to have genotype B than Han Chinese carriers, regardless of their HBeAg status. Since the mean age of aboriginal subjects was greater than that of Han Chinese subjects, age could be a confounding factor of this difference. However, when the analysis of the HBeAg status and genotype was adjusted for age, the prevalence of genotype B in aborigines was still significantly higher than that in Han Chinese in all age groups (Fig. 1A). In addition, the HBeAg-positive rate was significantly higher in Han Chinese who were younger than 50-year old (Fig. 1B).
| Han Chinese | Aborigines | P-value | |
|---|---|---|---|
| HBeAg-positive | n = 146 | n = 42 | <0.0001 |
| Genotype B | 65 (44.5%) | 35 (83.3%) | |
| Genotype C | 81 (55.5%) | 7 (16.7%) | |
| HBeAg-negative | n = 565 | n = 425 | <0.0001 |
| Genotype B | 452 (80.0%) | 398 (93.7%) | |
| Genotype C | 113 (20.0%) | 27 (6.4%) |
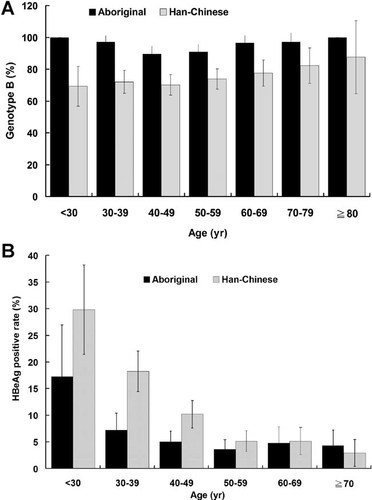
A: Prevalence of hepatitis B virus genotype B in Taiwanese aborigines and Han Chinese in different age groups. B: Rate of HBeAg-positive status in aborigines and Han Chinese in different age groups.
Comparisons of HBV Genotypes Between Aboriginal Language Groups Stratified by HBeAg
A comparison of the HBV genotypes in 467 aboriginal subjects from four different aboriginal language groups (Atayal, Paiwan, Tsou, and Bataan) who had complete genotype and HBeAg data showed that the overall prevalence of genotype B was highest in the Tsou group (98.2%), followed by the Paiwan (96.4%) and Atayal (88.1%) groups. The differences were statistically significant (P = 0.001); however, they were only found in HBeAg-negative subjects (Table V).
| Language groups | ||||
|---|---|---|---|---|
| Atayal | Paiwan | Tsou | P-value | |
| Overall | n = 218 | n = 194 | n = 55 | 0.001 |
| Genotype B | 192 (88.1%) | 187 (96.4%) | 54 (98.2%) | |
| Genotype C | 26 (11.9%) | 7 (3.6%) | 1 (1.8%) | |
| HBeAg-positive | n = 15 | n = 19 | n = 8 | 0.077 |
| Genotype B | 10 (66.7%) | 17 (89.5%) | 8 (100.0%) | |
| Genotype C | 5 (33.3%) | 2 (10.5%) | 0 (0.0%) | |
| HBeAg-negative | n = 203 | n = 175 | n = 47 | 0.0054 |
| Genotype B | 182 (89.7%) | 170 (97.1%) | 46 (97.9%) | |
| Genotype C | 21 (10.3%) | 5 (2.9%) | 1 (2.1%) | |
Clinical and Virological Characteristics of Subjects With HBV Genotype D
The HBeAg-positive rate and mean viral load of HBV DNA in the 13 subjects with HBV genotype D were similar to those in subjects with pure HBV genotype B. However, the percentage of subjects with genotype D and HBV DNA > 2,000 IU/ml was more similar to that in genotype C than genotype B. It is noteworthy that the birthplace and residence of the 13 subjects with HBV genotype D were clustered in southern Taiwan, and 12 of them were aborigines (Table VI).
| Male:female ratio | 6:7 |
| Age (years) | 49.3 (14.1) |
| HBeAg-positive status | 2/13 (15.4%) |
| AST (mean (SD)) (U/L) | 67.1 (96.9) |
| ALT (mean (SD)) (U/L) | 43.2 (31.1) |
| Log (HBV DNA) (mean (SD)) (IU/ml) | 5.4 (1.3) |
| α-Fetoprotein (ng/ml) | 11.1 (25.3) |
| History of blood transfusions | 2/13 (15.4%) |
| History of surgeries | 5/13 (38.5%) |
| Tattoos or body piercings | 5/13 (38.5%) |
| History of needle sharing | 0/13 (0.0%) |
| Residency in Pingtung County | 12/13 (92.3%) |
| Birthplace in Pingtung County | 12/13 (92.3%) |
| Member of Paiwan tribe | 10/13 (76.9%) |
DISCUSSION
This study found that Taiwanese aboriginal HBV carriers had fewer viral factors for developing HCC than Han Chinese carriers, such as lower HBeAg-positive rate and mean viral load and a higher prevalence of genotype B. Even after stratification by age, aborigines still had a lower HBeAg-positive rate and a higher percentage of genotype B infection in all age groups. These favorable viral factors may partly explain why Taiwanese aborigines have a higher HBV carrier rate but a lower HCC mortality rate than Han Chinese.
However, there are other possible explanations for the lower standardized mortality rate of HCC in Taiwanese aborigines, despite their higher standardized mortality rate of chronic liver diseases and cirrhosis compared with Han Chinese. For example, this difference might be due to comorbidity of HBV infection and alcoholic liver disease in aborigines. The mean ALT level in Han Chinese and aboriginal HBV carriers was similar. However, the higher mean serum level of AST and frequency of alcohol consumption in aborigines compared with Han Chinese implied that alcoholic liver disease might contribute to the higher standardized mortality rate of chronic liver diseases and cirrhosis in aboriginal HBV carriers [Shen et al., 1996]. In other words, due to their higher standardized mortality rate of chronic liver diseases and cirrhosis, aboriginal HBV carriers might not live long enough to develop HCC, which would reduce their standardized mortality rate of HCC. This hypothesis is supported by the observation that the mean life expectancy of Taiwanese aborigines and Han Chinese is 68.5 and 77.9 years, respectively (Council of Indigenous Peoples Taiwan, 2007). In addition, there might be host genetic variations, which are involved in the pathogenesis of HBV infection or disease progression, between these two groups. Further studies are needed to test this hypothesis.
The results of this study demonstrated that there are significant geographic differences in the distribution of HBV genotypes in Taiwan. Although HBV genotype B was the predominant genotype (80.6%) on the main island of Taiwan, which is consistent with previous findings [Kao et al., 2002], HBV genotype C was the predominant genotype (60%) on offshore islands. This difference can be explained by the fact that most of the ancestors of Taiwanese HBV carriers who live offshore originated from China where HBV genotype C is predominant [Liu et al., 2002].
In addition, there were ethnic differences in the distribution of HBV genotypes. The prevalence of HBV genotype B in Taiwanese aborigines was significantly higher than that in Han Chinese, regardless of the HBeAg status. Furthermore, the prevalence of genotype B in the Tsou tribe was higher than that in the Atayal tribe. In the past, aborigines could not travel easily between the main island of Taiwan and offshore islands or between different tribes. As a result, it is likely that the lack of gene flow between HBV carriers in these areas limited the evolution of HBV genotypes.
In this study, a small percentage of subjects were infected with HBV genotype D. Although HBV genotype D is common in Europe, India, and the Solomon Islands, it is very rare in Taiwan. Interestingly, these subjects were clustered in a small township in southern Taiwan and were predominantly from the Paiwan tribe. In addition, many had a history of tattoos, body piercings, or surgery. These similarities suggest a common source of HBV genotype D in these subjects. However, it is not known how they were infected. Additional epidemiological studies are being conducted to elucidate the possible routes of transmission and sources of infection in these carriers.
In conclusion, this study showed that Taiwanese aboriginal HBV carriers have lower viral load and HBeAg-positive rates and a higher prevalence of HBV genotype B compared with Han Chinese HBV carriers. These favorable viral factors may be partly responsible for the lower standardized mortality rate of HCC in Taiwanese aborigines. Further studies are needed to determine whether these results are applicable to other Austronesian language groups in the world.
Acknowledgements
We thank Associate Professor Chun-Jen Liu and Miss Ting-Chih Chen for their technical assistance.




