Molecular and clinical effects of betamethasone in human t-cell lymphotropic virus type-i-associated myelopathy/tropical spastic paraparesis patients†
Carolina Alberti and Luis Cartier equally contributed to this work.
Abstract
There is no effective therapy for human T-cell lymphotropic virus type I (HTLV-I)-associated myelopathy/tropical spastic paraparesis (HAM/TSP). Glucocorticoids are effective to reduce the motor disability in these patients, but its role as anti-spastic drugs is unknown. Here it is reported the use of corticosteroids in HAM/TSP. The goal was to find reliable molecular markers linked to treatment effectiveness. The clinical efficacy of corticosteroids was studied in 22 HAM/TSP. The treatment was a single dose of 7.0 mg of systemic betamethasone. Pre-treatment samples were obtained immediately before steroid administration and post-treatment samples were collected after 5 days. Neurological disability was evaluated by the Osame's Motor Disability Scales. Relative levels of Tax, Foxp3, IL-10, TGF-β, CTLA-4, and GITR mRNA were measured and the percentage of CD4+Foxp3+ and CD4+Tax+ populations was quantified in PBMCs by real-time PCR and flow cytometry, respectively. The same parameters were studied in eight untreated carriers. Betamethasone treatment showed neurological improvement in 21 HAM/TSP patients, with one patient without response to treatment. This therapy was associated with a decrease in Tax mRNA load and CD4+Tax+ T cells in HAM/TSP. Simultaneously, an increase in Foxp3 mRNA and CD4+Foxp3+ T cell was detected in these patients. The other markers studied had no significant changes after treatment. Clinical improvement in betamethasone-treated HAM/TSP was associated with an inverse relationship between a decrease in Tax and an increase in Foxp3 at the mRNA and protein levels. These results suggest that both Tax and Foxp3 may represent potential biomarkers for drug treatment assessments in HAM/TSP. J. Med. Virol. 83:1641–1649, 2011. © 2011 Wiley-Liss, Inc.
INTRODUCTION
Human T-cell lymphotropic virus type I (HTLV-I) is the etiologic agent of HTLV-I-associated myelopathy/tropical spastic paraparesis (HAM/TSP) [Osame et al., 1986]. This progressive spastic paraparesis without remittances is interpreted as an inflammatory disease [Nakagawa et al., 1995; Uchiyama, 1997], and also as a neurodegenerative disease [Cartier et al., 1997, 2007; Liberski et al., 1999]. Nevertheless, the progression of the disease is still unknown [Oh et al., 2006]. Worldwide the majority of infected individuals remain asymptomatic carriers while approximately 0.25–3.0% of those infected develop HAM/TSP [Kaplan et al., 1990]. There are no effective ways to prevent the development of HAM/TSP. Glucocorticoids are effective to reduce the motor disability in these patients, probably because of their anti-inflammatory properties [Gotuzzo et al., 2004; Verdonck et al., 2007].
The anti-inflammatory and immunosuppressive effects of the corticoids are widely known, but the anti-spastic action of these drugs is less mentioned [Cartier et al., 1977; Cartier and Verdugo, 1988]. Based on the last effect, corticoids have been used to obtain an effective decrease of spasticity in HAM/TSP patients. The repeated use of corticosteroids allowed the selection of 7.0 mg of rapid and slow action betamethasone in a single-dose monthly as a suitable treatment. This small dose of the drug produces defined improvements, increasing the step-length, speed, gait, and balance in HAM/TSP patients; effects on the control of spastic bladder are seen as well. Additionally, patients in long-term treatment show a slower functional deterioration compared with untreated patients [Cartier, 1998]. As a result of these findings, factors that could be involved in this functional improvement were studied.
In HAM/TSP, natural regulatory T cells (nTreg) characterized by the markers CD4+CD25hiFoxp3+ are the main reservoir of the virus in vivo [Yamano et al., 2004, 2005]. These cells have the function to suppress, through cell–cell contact, the response of effector T cells and antigen-presenting cells in chronic diseases, including those caused by retroviruses [Grant et al., 2006; Roncarolo and Gregori, 2008]. Tax is a viral and cellular transcriptional regulator associated with the progression of HAM/TSP in most cases [Uchiyama, 1997; Nagai and Jacobson, 2001]. In vitro studies have demonstrated a decrease in Foxp3 levels in the presence of Tax protein [Yamano et al., 2004]. The mechanism of this regulation and its implication in the progression of HAM/TSP are still unknown [Yamano et al., 2005; Toulza et al., 2008].
The aim of this work was to find reliable molecular markers for the effectiveness of betamethasone treatment. In order to characterize the immunological and neurological effects of betamethasone in HAM/TSP patients, this study was directed to evaluate the variation of cytokine levels and receptors involved in down-regulate the immune-response as well as the neurological improvement reflected in gait commitment. In this study, a clinical improvement of HAM/TSP patients treated with betamethasone is reported. This improvement is associated with a characteristic molecular pattern of immunological and viral markers. A clear inverse relationship between a decrease in Tax viral protein and an increase in nTreg marker Foxp3 in response to treatment was found. Other immunological markers such as IL-10, TGF-β, CTLA-4, and GITR were also studied to estimate the involvement in immuno-modulator processes, but no correlation was found either between markers or between markers with gait improvement.
MATERIALS AND METHODS
Patients and Healthy Control Subjects
All the experiments were performed in compliance with relevant laws and the University of Chile Ethics Committee guidelines and in accordance with the ethical standards of the Declaration of Helsinki. Informed consent was obtained from all individuals. HAM/TSP patients fulfilled criteria of gait commitment according to The World Health Organization. Patients were evaluated clinically before and after therapy. Motor disability was evaluated by neurologists in each patient visit. Motor dysfunction was evaluated on the basis of the Osame's Motor Disability Score, in which motor dysfunction is graded on the scale from 0 (normal gait and running) to 13 (completely bedridden). EDTA-treated blood was obtained from 22 HAM/TSP patients, 8 asymptomatic carriers (Carriers), and 8 healthy non-infected subjects. All patients were treated with systemic betamethasone in a single dose of 3.0 mg of rapid action betamethasone plus 4.0 mg of slow action betamethasone. Pre-treatment samples were obtained immediately before steroid administration and post-treatment samples were collected after 5 days. Table I shows clinical data of HAM/TSP patients before and after treatment. Carriers were used as a reliable infected control group without neurological manifestations, and healthy non-infected donors were used as non-infected controls. Both control groups were not treated with betamethasone. The Carriers group was characterized by absence of motor disability, a male:female ratio of 1:1 and a mean age of 37.3 ± 10.2. The healthy non-infected group was similar but with a mean age of 32.7 ± 13.6.
| Patients | Age | Sex | Disease evolution (years) | OMDS before treatment | OMDS after treatment |
|---|---|---|---|---|---|
| 1 | 40 | F | 3 | 4 | 3 |
| 2 | 46 | F | 3 | 5 | 4 |
| 3 | 68 | F | 14 | 4 | 3 |
| 4 | 74 | F | 9 | 5 | 4 |
| 5 | 63 | F | 12 | 5 | 4 |
| 6 | 69 | F | 12 | 5 | 4 |
| 7 | 61 | M | 17 | 5 | 4 |
| 8 | 56 | M | 7 | 6 | 5 |
| 9 | 53 | M | 5 | 4 | 3 |
| 10 | 71 | F | 17 | 7 | 6 |
| 11 | 74 | F | 20 | 5 | 4 |
| 12 | 79 | M | 25 | 4 | 2 |
| 13 | 54 | M | 12 | 9 | 8 |
| 14 | 61 | F | 16 | 7 | 7 |
| 15 | 36 | F | 11 | 6 | 4 |
| 16 | 50 | F | 10 | 5 | 4 |
| 17 | 57 | F | 10 | 3 | 2 |
| 18 | 50 | M | 18 | 4 | 3 |
| 19 | 72 | F | 12 | 5 | 4 |
| 20 | 49 | F | 24 | 7 | 6 |
| 21 | 63 | F | 14 | 4 | 3 |
| 22 | 65 | F | 15 | 5 | 4 |
- Each column shows different parameters related with age, sex, and years of disease evolution. Motor disability stage measured as gait commitment was evaluated before treatment and 5 days after betamethasone administration.
- OMDS, Osame's Motor Disability Scales.
PBMCs Isolation
Peripheral blood mononuclear cells (PBMCs) were obtained from 10 ml of EDTA-treated blood by Ficoll–Hypaque density gradient centrifugation; they were washed three times with phosphate-buffered saline (PBS). The number of PBMCs collected varied between 7 × 106 and 10 × 106.
Nucleic Acid Isolation and cDNA Synthesis
RNA was isolated with RNeasy kit (Qiagen, Valencia, CA) from PBMCs according to the manufacturer's protocol. Reverse transcription was performed with Taq-Man reverse transcription in a one-step PCR.
Relative Real-Time PCR
cDNA was synthesized for relative quantitation of Tax, CTLA-4, GITR, IL-10, TGF-β, and Foxp3 transcripts, after and before betamethasone treatment of HAM/TSP patients. Samples from Carriers and non-infected healthy donors were also analyzed. Hypoxanthine ribosyltransferase (HPRT) was used as housekeeping gene. We designed Tax, Foxp3, HPRT, and GITR primers using AmplifiX 1.4 software based on sequences reported in GeneBank; Tax: forward primer (5′-ATC CCG TGG AGA CTC CTC AA-3′), reverse primer (5′-CCA AAC ACG TAG ACT GGG TAT CC-3′); GITR: forward primer (5′-CGA GGA GTG CTG TTC CGA GT-3′), reverse primer (5′-TGG AAT TCA GGC TGG ACA CAC-3′); Foxp3: forward primer (5′-AAT GGC ACT GAC CAA GGC TTC ATC T-3′), reverse primer (5′-GTG CCT CCG GAC AGC AAA CA-3′); and HPRT: forward primer (5′-TGC TGA GGA TTT GGA AAG GGT GTT-3′), reverse primer (5′-AGC ACA CAG AGG GCT ACA ATG TGA-3′). Primers for CTLA-4, IL-10, and TGF-β were described previously [Boeuf et al., 2005; Schaub et al., 2006]. PCR products were spanning exon–intron borders in order to avoid amplification of contaminant genomic DNA. cDNA was amplified using Brilliant® II SYBR® Green master mix (Stratagene, Agilent Technologies, Wilmington, DE). When possible, amplifications were carried out in duplicates. Analysis of melting curves showed a single pick for each marker amplified, coincident with the size of PCR products analyzed in agarose gels. Relative quantitation was made with the comparative threshold cycle (ΔΔCT) formula with HPRT as endogeneous housekeeping gene. Carriers were used as control group for calculation of ΔΔCT, having previously compared them with healthy non-infected individuals. It was not possible to compare Tax mRNA levels in both groups because of the lack of Tax gene in healthy non-infected individuals. Data were normalized to the average value of Carriers.
Flow Cytometry
PBMCs for flow cytometry were cultured 14 h in RPMI 1640 (Gibco, Paisley, UK) supplemented with 10% fetal bovine serum (Gibco) and 20 nM Concanamycin A (Sigma–Aldrich, St Louis, MO) in order to inhibit the action of CD8+ cytotoxic T lymphocytes. Cells were harvested and stained with fluorophore-conjugated antibodies against the following antigens: CD4-FITC (BD Biosciences, San Jose, CA), Foxp3-PE (BD Biosciences) and Tax-APC, kindly provided by Dr. Yuetsu Tanaka. For nuclear Foxp3 and Tax staining, cells were permeabilized with fixation and permeabilization reagents (eBiosciences, San Diego, CA). Matched isotype controls were used at the same concentration as the respective antibodies. A three-color flow cytometry in a FACS-CANTO instrument (Beckton Dickinson) was performed; WinMDI 2.9 software was used for data analysis. To calculate mean fluorescence intensity (MFI) the MFI software (University of Massachusetts) was used. Mean fluorescence of each marker over CD4+ subpopulation was used to determine the level of Foxp3 and Tax protein. Values of MFI plotted were divided by 100 to keep the same scale range of the mRNA data.
Statistics
Statistical analysis was made with Graph Pad Prisma 5.0. Data were verified for Gaussian distribution. For evaluating betamethasone effects in HAM/TSP patients, data before and after treatment were compared using Wilcoxon-singed rank test. Kruskal–Wallis test was used for calculation of differences between independent groups. Data are shown as mean ± SD. The Pearson's correlation was used to evaluate relationship between mRNA and protein levels and between cell populations percentage. Differences in P-values of 0.05 or less were considered significant.
RESULTS
Effects of Betamethasone in Relative Expression of Foxp3 and Tax mRNA in PBMCs of HAM/TSP Patients
A significant increase in the relative amounts of Foxp3 mRNA in HAM/TSP patients treated with systemic betamethasone was observed, compared with pre-treatment samples from the same individuals. There was also a statistical difference between amounts of Foxp3 mRNA from pre-treatment patients and Carriers, but not between healthy non-infected individuals and Carriers, or post-treatment samples (Fig. 1A). Samples from 21 HAM/TSP patients treated with betamethasone showed significant decreases in Tax mRNA compared with those from pre-treatment controls (Fig. 1B). Pre-treatment samples of HAM/TSP showed significant increases compared with those from Carriers. Furthermore, post-treatment HAM/TSP did not show differences from the carriers group. Thus, betamethasone decreased Tax mRNA close to the amounts seen in Carriers. Only patient 14 did not show changes in Tax mRNA levels before and after treatment. Tax mRNA levels were low and similar with those from Carriers.
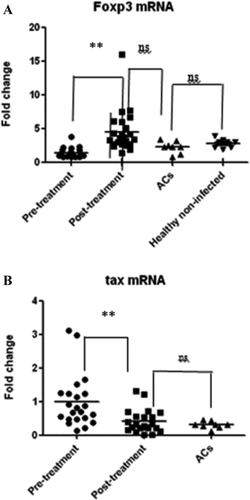
Fold change of Foxp3 and tax mRNA in HAM/TSP patients treated with betamethasone. A: Patients treated with betamethasone showed significant differences in Foxp3 mRNA amounts (4.315 ± 0.73) compared to their pre-treatment condition (1.368 ± 0.16; **P < 0.0032). Carriers did not reveal significant differences compared to healthy non-infected controls, as well as with post-treatment samples. B: Betamethasone produced a decrease in relative amounts of tax mRNA in HAM/TSP patients (0.469 ± 0.09), in relation to pre-treatment samples (1.039 ± 0.18; **P < 0.0045). Relative quantitation of mRNA in Carriers (0.308 ± 0.038) did not show significant differences with post-treatment samples.
No significant differences were found in the amounts of mRNA of the immunological markers CTLA-4, GITR, IL-10, and TGF-β in PBMCs from HAM/TSP patients before and after betamethasone treatment, compared with Carriers. There were no statistical differences between these two groups in all analyzed markers (data not shown).
Effect of Betamethasone Treatment in CD4+Foxp3+ and CD4+Tax+ Cell Population in HAM/TSP Patients
Betamethasone treatment led to a significant increase in the CD4+Foxp3+ cell population in 21 HAM/TSP patients compared with pre-treatment controls (Fig. 2A and B). Patient 14 showed a decrease from 2.5 to 1.9% in CD4 +Foxp3+ cells in pre-treatment and post-treatment samples, respectively. However, the relative amount of Foxp3 mRNA remained similar before and after treatment. No significant differences between the percentages of CD4+Foxp3+ in carriers, healthy non-infected and HAM/TSP patients post-treatment were observed. Inverse results in the CD4+Tax+ cell population in response to betamethasone compared to those of CD4+Foxp3+ cells were found. There was a significant decrease in the percentage of CD4+Tax+in 21 HAM/TSP-treated patients compared with non-treated controls (Fig. 3A and B). Only patient 14 did not show changes in the percentage of CD4+Tax+ cells, with similar levels as those of asymptomatic carriers characterized by very low levels of CD4+Tax+. A comparison of this cell population between Carriers and HAM/TSP-treated patients did not show statistically significant differences. A correlation between CD4+Foxp3+ and CD4+Tax+ population before treatment did not show a statistically significant result (data not shown). When data of both cell populations after betamethasone treatment were plotted together, the result was a negative and statistically significant correlation (Pearson's r = −0.56; Fig. 4A).
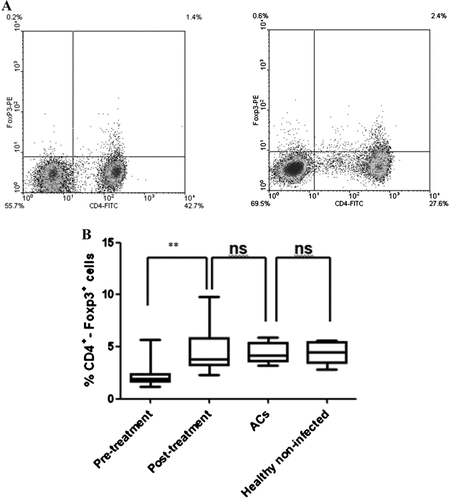
Flow cytometry analysis of CD4+Foxp3+ population in HAM/TSP patients treated with betamethasone. Percentage of Treg population was obtained from PBMCs collected from HAM/TSP patients before treatment and 5 days after betamethasone administration. Data were also compared with PBMCs from Carriers and healthy non-infected controls. A: Dot-plot representation of CD4+Foxp3+ population obtained from a pre-treatment sample (left) and post-treatment sample (right). B: Comparison of results from different groups analyzed. Significant differences were found between pre-treatment (2.33 ± 1.24) and post-treatment (5.01 ± 2.24) conditions (**P < 0.0036). Post-treatment patients did not show significant differences compared to Carriers (4.34 ± 1.01) and healthy non-infected controls (4.44 ± 1.03).
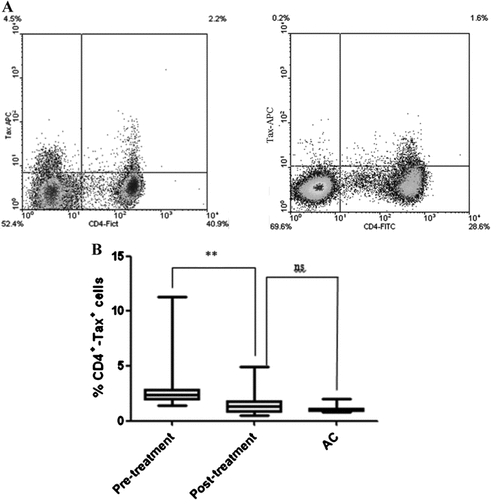
Quantitation of CD4+Tax+ population in HAM/TSP patients under betamethasone treatment. PBMCs obtained from HAM/TSP patients before and after treatment as well as from Carriers, were used to quantify CD4+Tax+ cells and to evaluate the effect of systemic betamethasone in this population. We used Carriers as control group, considering that their CD4+Tax+ levels are very low and manifestation of motor disability are absent in this individuals. A: Dot-plot analysis of a pre-treatment sample (left) and post-treatment sample (right) highlighting the percentage of CD4+Tax+ cells. B: Results show a statistical difference between pre-treatment (3.03 ± 2.79) and post-treatment (1.43 ± 1.23) conditions (**P < 0.0038). No statistical differences were observed when post-treatment and Carriers individuals were compared.
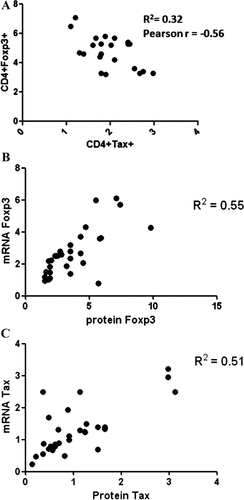
Correlation between Foxp3 and Tax in HAM/TSP patients. A correlation between CD4+Tax+ and CD4+Foxp3+ post-treatment percentage is shown. Data obtained from pre-treatment and post-treatment samples were plotted together in a correlation graph of mRNA and protein levels as well. A: A negative and statistically significant correlation between CD4+Foxp3+ and CD4+Tax+ cell population was found in post-treatment samples (r = −0.56; P < 0.01). B: Results of Foxp3 mRNA and protein levels calculated as MFI in flow cytometry data showed a positive and statistical significant correlation (r = 0.55; P < 0.001). C: A positive and statistical significant correlation was also found in the case of Tax protein and mRNA data (r = 0.51; P < 0.001).
Correlation of mRNA Expression and Protein Levels of Foxp3 and Tax
To support the findings at the mRNA level related with Foxp3 and Tax levels, a correlation between protein and mRNA for each marker was made. Foxp3 and Tax showed both a positive and statistically significant correlation between mRNA expression and protein levels in HAM/TSP patients before and after treatment (Fig. 4B and C). Tax and Foxp3 mRNA of PBMCs from patients before treatment did not show a statistically significant correlation. Instead, the evaluation of Tax and Foxp3 mRNA after the treatment revealed a significant negative correlation with a Pearson's r = −0.55 (data not shown).
DISCUSSION
Effective treatment strategies for HAM/TSP are still a challenge and clinical studies available are not enough to support their therapeutic effects. Most therapies are symptomatic, being focused on reducing the inflammatory response in affected tissues. Interferon-α (IFN-α), which has both cytostatic and antiviral activity has been used as a potential therapy for HAM/TSP, but with modest results in reducing HTLV-I proviral load [Izumo et al., 1996; Nakagawa et al., 1996; Feng et al., 2003, 2004]. This decrease would be associated with changes in the number of CD8+ T cells [Saito et al., 2004]. Alternative treatments such as oral prednisolone, intrathecal hydrocortisone [Kira et al., 1991; Araujo et al., 1995; Nakagawa et al., 1996], plasmapheresis, and intravenous gammaglobulin have been used [Matsuo et al., 1988; Kuroda et al., 1991; Gold et al., 2007]. Nevertheless, they have not shown clear beneficial effects. The use of antiretroviral drugs including zidovudine and lamivudine has not reported clinically significant changes in 16 patients in a randomized, double-blind study [Taylor et al., 2006]. Even though other kinds of glucocorticoids have been used in HAM/TSP, the advantages of the treatment presented in this work are based on the following characteristics: (a) betamethasone has no interaction with mineralocorticoid receptors, thus there is a higher concentration interacting with glucocorticoid receptors compared with other drugs [Habib and Safia, 2009]; (b) the equivalent dosage compared with other glucocorticoids is eight times less; (c) patients treated with betamethasone do not develop hypertension and features related with this condition; (d) betamethasone has the longest half-life compared with other glucocorticoids; (e) the drug formulation with phosphate and acetate salts increases its bioavailability; (f) a single unique dose is effective for ameliorate disease symptoms; (g) patients do not show secondary effects related with corticoid usage like polyuria, polydipsia, and polyphagia (NIH Clinical Trials). These data were obtained from experimental database (http://www.cancer.gov/Search/ClinicalTrialsLink.aspx?id=39273&idtype=1” active clinical trials) and from clinical observation of patients in this study.
The action of glucocorticoids as immune-modulators in chronic diseases by increasing Foxp3 is well documented [Karagiannidis et al., 2004; Braitch et al., 2009]. It has been suggested that HAM/TSP patients present a reduced Foxp3-dependent suppression capacity [Grant et al., 2008]. This study showed that HAM/TSP patients treated with betamethasone exhibit an increase in Foxp3 mRNA and CD4+Foxp3+ T cell population at levels comparable with those of non-infected individuals and Carriers. It remains to establish if glucocorticoids-dependent Foxp3 up-regulation leads to a recovery in Treg function compared to Treg obtained from pre-treated HAM/TSP patients. Foxp3 regulatory properties only become active if the immunological environment lacks of danger signals or immune responses are exhausted [Karagiannidis et al., 2004]. Thus, the relatively high levels of pro-inflammatory cytokines or inflammatory-signals present in HAM/TSP might explain the Treg response observed in these patients. A positive correlation was observed between the mRNA expression and protein levels of Foxp3 after betamethasone treatment. Glucocorticoids action on HTLV-I viral component has not been reported.
Tax protein is the major viral component associated with the development and progression of HAM/TSP, being its mRNA identified as one of the best biomarker of this disease [Yamano et al., 2002; Oh and Jacobson, 2008]. An association was found between betamethasone therapy and a significant decrease in both CD4+Tax+ T cell population and Tax mRNA in treated patients compared with the pre-treated condition. The results show that HAM/TSP patients treated with betamethasone have reduced levels of infected T CD4+ cells and Tax mRNA with no significant differences with Carriers. Thus, carriers could be a suitable group for comparing the effectiveness of betamethasone treatment in HAM/TSP patients in terms of Tax levels and clinical improvement. In treated patients the decrease in the number of CD4+Tax+ cells is inversely proportional to the increase of CD4+Foxp3+ T cells. Only a single patient did not follow this trend not showing a clinical improvement after treatment. This patient had very low percentage of CD4+Tax+ T cells and Tax mRNA, similar with those detected in Carriers. This patient showed a slight decrease of CD4+Foxp3+ cells but Foxp3 mRNA levels did not change with betamethasone treatment. These results suggest that the increase in Foxp3 mRNA and decrease of Tax mRNA are simultaneously required for clinical improvement of HAM/TSP patients.
In vitro studies showed a Tax-dependent CD4+CD25+ Tregs reduction through the suppression of Foxp3 expression [Yamano et al., 2005]. Repression of Tax transcription and transactivation functions by Foxp3 through targeting both NF-κB and CREB pathways have been published [Grant et al., 2006]. Based on the results, betamethasone might employ the same mechanism to reduce Tax levels. Glucocorticoids inhibit this pathway to shut down the immune response. Therefore, betamethasone therapy could produce both an increase in Foxp3 levels and a repression of Tax transcriptional pathways, with the concomitant reduction of this viral protein. Tax and Foxp3 are suggested as potential biomarkers for assessing treatment in HAM/TSP.
No significant differences in mRNA levels of the immunological markers CTLA-4, GITR, IL-10, and TGF-β in HAM/TSP were found in treated patients. These results suggest that cytokines related with the inducible Treg population are not involved in glucocorticoid-dependent Foxp3 increase.
Since all HAM/TSP patients, with one exception, showed gait recovery after glucocorticoid treatment, these results suggest that glucocorticoids might be able to produce both inmunological and neurogical changes by increasing Treg population and decreasing the spasticity condition, respectively. Although corticosteroids have been the most widely used therapy for HAM/TSP, few clinical trials of them have been recently published [Izumo et al., 1996; Croda et al., 2008]. These were designed as uncontrolled case series, showing that this therapy appears to be clinically beneficial with a transient effect. The role of glucocorticoids as anti-spastic drugs was unknown. The beneficial effects of corticosteroids in HAM/TSP could be due to IFN-γ overproduction [Casseb and Penalva, 2000]. Anti-inflammatory properties of corticosteroids might have an impact on myelin membrane inflammation process observed in HAM/TSP, mainly in those with few years of disease when inflammation is more prominent [Araujo et al., 1995].
The results of this study should be interpreted with caution, since we performed a short clinical trial, unblinded and not placebo controlled. Consequently, for clinical trial in HAM/TSP is important to know the impact of epidemiological variables of patients (e.g., age, duration of symptoms, age of onset, disability scores, time of progression, etc.), and the establishment of biomarkers to assess the effectiveness of treatment. Thus, only a double-blinded clinical trial, and placebo-controlled study could ultimately determine the role of corticosteroids in HAM/TSP.
Taken together, the findings suggest a dual action of glucocorticoids. An immunological action reflected by a concomitant increase in Foxp3 and decrease of Tax and a neurological action determined by a reduction of spasticity in HAM/TSP patients. Further research on pathways leading to Tax repression as consequence of glucocorticoids therapy and the effect of this drug on the central nervous system, could help to understand the mechanisms related to betamethasone action in the context of HAM/TSP.
Acknowledgements
We thank Dr. Christopher I. Pogson for the critical reading of the review.




