T1846 and A/G1913 are associated with acute on chronic liver failure in patients infected with hepatitis B virus genotypes B and C†
This experiment was approved by the ethics committee of 302 Military Hospital.
Abstract
The aim of this study was to determine whether mutations in the hepatitis B virus (HBV) genome are associated with the onset of acute on chronic liver failure (ACLF). For the longitudinal study, full-length HBV genomes were cloned and sequenced from four ACLF patients and compared with sequences from matching samples collected before ACLF. For the cross-sectional study, 166 serum samples were obtained, including 49 samples from patients with ACLF. The results of longitudinal study showed that C53T, A1846T, and G1896A were the most common mutations in association with ACLF. In the cross-sectional study 61.2% patients with ACLF presented with T1846, which was higher than patients with chronic hepatitis B (CHB) (11.1%), liver cirrhosis (LC) (31.1%), and hepatocellular carcinoma (HCC) (33.3%). Prevalence of A/G1913 was 42.9% in patients with ACLF, also higher than patients with CHB (2.2%), LC (17.8%), and HCC (11.1%). There were no differences in HBV genotype and patients' HBeAg status among patients with ACLF, LC, and HCC. However, prevalence of T1846 was much higher in patients infected with genotype B (57.1%) than genotype C (30.4%). A/G1913 was higher in HBeAg negative patients (28%) than HBeAg positive patients (13.2%). Results of a multivariable analysis showed that T1846 and A/G1913 were independent factors for ACLF (OR = 3.373 and 4.244, respectively). Interestingly, T1846 destroys an ATG codon of a small open reading frame in the preC region, which may increase core protein expression. We conclude that T1846 and A/G1913 in the preC/C gene are closely associated with ACLF. J. Med. Virol. 83:996–1004, 2011. © 2011 Wiley-Liss, Inc.
INTRODUCTION
Chronic hepatitis B virus (HBV) infection is associated with a wide spectrum of disease manifestations from asymptomatic infection to severe liver diseases including liver cirrhosis (LC), hepatocellular carcinoma (HCC), and liver failure. HBV belongs to the family Hepadnaviridae. It is a small DNA virus with compact genomic organization encoding four sets of gene products and its replication involves an RNA intermediate. The high error rate associated with the reverse transcription step of HBV replication generates many mutations, a small fraction of which is selected during different stages of infection or in response to therapy because they facilitate evasion of the host immune response, confer resistance to antivirals, or cause exacerbation of the disease [Bartholomeusz and Locarnini, 2001; Jalan and Williams, 2002; Sen et al., 2002].
HBV-related acute on chronic liver failure (ACLF) is a major cause of death for patients with chronic HBV infection. This condition was only recently identified by the Asian Pacific Association for the Study of the Liver (APASL). A cross-sectional study suggested the possible involvement of BCP and preC mutations in ACLF [Ren et al., 2010]. Such mutations are also associated with severe forms of chronic liver disease elsewhere according to earlier studies [Bartholomeusz and Locarnini, 2001]. Most studies have performed horizontal comparison of fulminant hepatitis or liver failure with control cases, mainly in CHB patients [Hou et al., 2002; Ren et al., 2010]. Some BCP mutations such as A1762T and G1764A were more prevalent in liver failure than in CHB, but accumulation of these mutations is apparently time-dependent. Indeed, studies showed that their incidences to be highest in patients with HCC [Chen et al., 2008; Fang et al., 2008]. To assess the clinical significance of HBV genotype and mutations in the HBV genome on ACLF, in the present study longitudinal analysis was performed to identify mutations that arise at the onset of ACLF. In addition, a large number of patients with CHB, LC, HCC, and ACLF were compared for cross-sectional analysis.
MATERIALS AND METHODS
Study Subjects
All the specimens used in the present study were derived from the Serum Bank of the 302 Military Hospital. All the recruited cases were from hospitalized patients, whose history, serum sampling times, and results of accessory examinations were fully recorded. The patients were diagnosed for chronic HBV infection by history and laboratory examinations as having a course of HBV infection greater than 6 months. The diagnostic criteria of ACLF were as defined by APASL: acute hepatic insult manifesting as jaundice [serum bilirubin ≥5 mg/dl (85 µM)] and coagulopathy (INR ≥1.5 or prothrombin activity <40%), complicated within 4 weeks by ascites and/or encephalopathy in patients with previously diagnosed or undiagnosed chronic liver diseases [Sarin et al., 2009]. The clinical diagnosis of CHB and LC conformed to the 2000 Xi An criteria [Chinese Society of Infectious Diseases and Parasitology and the Chinese Society of Hepatology of the Chinese Medical Association, 2000]. Three independent criteria were used for HCC diagnosis: positive pathological results, typical images compatible with HCC, and/or α-fetoprotein ≥400 ng/ml. In all patients, co-infection with hepatitis A, C, D, and E viruses were excluded, and co-infection with cytomegalovirus, Epstein–Barr virus, and human immunodeficiency virus was also excluded by negative serum tests of IgM antibodies. Concurrent conditions of drug-related hepatitis, autoimmune hepatitis, fatty liver, alcoholic hepatitis, obstructive jaundice, and hereditary and metabolic hepatitis were ruled out as well.
Twenty-one patients with ACLF were enrolled in the longitudinal analysis. They met the following additional criteria: availability of serum samples at the time of diagnosis of ACLF and at least 1 year earlier, and positive HBV DNA tests at both times of sampling. Only 4 of the 21 patients showed positive amplification of the full-length HBV genome by a single round of PCR for both serum samples. All (P1–P4) died from ACLF and associated complications: P1 and P3 from gastrointestinal hemorrhage, P2 from hepatorenal syndrome, and P4 from hepatic encephalopathy. The two control patients for the longitudinal study (P5 and P6) were diagnosed with LC, and the two samples used for PCR amplification correspond to the LC and CHB stages, respectively (Table I). None of the patients had any history of antiviral treatment with interferon or nucleos(t)ide analogs.
| Patient | Data of serum samples collection | Sex | Age (year) | T.BIL (µmol/L) | ALT (U/L) | PA (%) | HBV DNA (log value) | Genotype | HBeAg | Diagnosis |
|---|---|---|---|---|---|---|---|---|---|---|
| P1 | 05/24/2005 | M | 34 | 66.8 | 36 | 32 | 4.3 | C | + | LC |
| 12/16/2006 | 246.1 | 41 | 16 | 5.5 | C | − | ACLF | |||
| P2 | 07/03/2002 | F | 48 | 29.5 | 53 | 54 | 4.1 | C | + | LC |
| 12/26/2005 | 430.1 | 41 | 20 | 5.3 | C | − | ACLF | |||
| P3 | 08/16/2006 | F | 60 | 36 | 32 | 55 | 5.2 | C | − | LC |
| 08/05/2008 | 164 | 112 | 28 | 5.7 | C | − | ACLF | |||
| P4 | 06/14/2005 | F | 63 | 14.4 | 487 | 73 | 6.0 | C | − | LC |
| 08/20/2006 | 314 | 24 | 5 | 7.6 | C | − | ACLF | |||
| P5 | 10/27/2003 | M | 31 | 23.1 | 722 | 78 | 7.2 | C | + | CHB |
| 12/19/2007 | 45.4 | 85 | 58 | 5.8 | C | + | LC | |||
| P6 | 04/19/2004 | M | 31 | 20 | 38 | 70 | 6.2 | C | − | CHB |
| 03/19/2007 | 35 | 104 | 70 | 5.5 | C | − | LC |
- T.BIL, total bilirubin; PA, prothrombin activity.
One hundred sixty-six serum samples from patients with chronic HBV infection were used for cross-sectional analysis: 49 with ACLF, 45 with CHB, 45 with LC, and 27 with HCC. Except for primary diagnosis, the staff did not have access to patient name, gender, age, and laboratory test results during the course of serum sample selection.
Laboratory Examinations
Upon hospitalization, the patients underwent routine liver function test, as well as tests of renal function, complete blood count, serology (HBsAg, HBeAg/anti-HBe), HBV DNA, and HBV genotyping. HBsAg and HBeAg/anti-HBe were detected by ELISA (Kewei Diagnostic Ltd., Beijing, China) or chemiluminescence (Abbott Laboratories, Chicago, IL). The HBV DNA level was determined using a quantitative fluorescent PCR kit (Fuxing Clone Co., Shanghai, China) with a detection limit of 500 copies/ml (approx. 100 IU/ml). The surface gene (nt 256–796) was used to determine HBV genotype as described previously [Lindh et al., 1997].
PCR, Cloning, and Sequencing of Complete HBV Genome for the Longitudinal Study
HBV DNA was extracted from 200 µl serum sample using the QIAamp DNA Blood Mini Kit. A single round of PCR was employed to amplify the complete HBV genome using the Expand High Fidelity PCR System (Roche, Mannheim, Germany) with previously described primer pairs [Tran et al., 2008]: HBV-P1, 5′-CCG GAA AGC TTA TGC TCT TCT TTT TCA CCT CTG CCT AAT CAT C-3′ (underlined: HindIII site); and HBV-P2 5′-CCG GAG AGC TCA TGC TCT TCA AAA AGT TGC ATG GTG CTG GTG-3′ (underlined: SacI site). The PCR product was separated on agarose gel and purified with a QIAquick Gel Extraction Kit. The purified product was double digested with HindIII and SacI, and inserted into PCR2.1 vector (Invitrogen, Carlsbad, CA). The ligation product was transformed into high efficiency JM109 competent cells (Promega (Beijing) Biotech Co., Ltd., Beijing, China), and three or four clones were selected for plasmid DNA preparation. The nucleotide sequences of the cloned HBV DNA were determined with an ABI PRISM 3730xl DNA Analyzer using BigDye terminator v3.1.
PCR and Sequencing of the BCP/preC/C Region for Cross-Sectional Analysis
A DNA segment covering the BCP and preC/C regions was amplified by PCR in a 50 µl volume, using 15 µl extracted DNA, primers P1 (5′-TCG CAT GGA GAC CAC CGT GA-3′, nt 1604–1623) and P2 (5′-ATA GCT TGC CTG AGT GC-3′, nt 2076–2060). Twenty-two of 166 samples failed to yield a distinct band, and a second round of PCR with nested primers was performed. To this end, 1 µl of the PCR product was re-amplified with primers P3 (5′-CAT AAG AGG ACT CTT GGA CT-3′, nt 1653–1672) and P4 (5′-GGA AAG AAG TCA GAA GGC-3′ nt 1974–1957). Cross-contamination was prevented by necessary precautions and negative controls were included in each assay. The PCR products were purified by agarose gel electrophoresis and nucleotide sequences were determined.
Sequence Comparison and Statistical Analysis
The Vector NTI Suite 9.0 software was used to align nucleotide sequences and Stata v.11 was used for all data analysis. Three or four PCR clones of each sample in longitudinal analysis were aligned to identify the dominant species that was used for alignment. All complete HBV genome sequences were used for phylogenetic analysis. The final unrooted phylogenetic tree picture was drawn with MEGA 4.1 by the neighbor-joining method, and bootstrap value was set at 1,000 replicates. Parameters with normal distribution, such as age and HBV DNA, were expressed as mean ± standard deviation, and were compared using one-way ANOVA tests among multiple samples and with Student's t-test between two samples. Parameters with non-normal distribution, such as levels of bilirubin, ALT, and PA, were expressed as median (min–max) and compared using a Kruskal–Wallis test among multiple samples and with the Mann–Whitney U-test between two samples. Categorical data were compared with a χ2 test. A P-value less than 0.05 was considered statistically significant. Analyses of the association of HBV mutations and onset of ACLF were performed by bivariate logistic regression. Variables with a statistically significant association (P < 0.05) were included in multivariable models.
RESULTS
Definition of Mutations
The availability of two serum samples for the longitudinal study makes the definition of mutations straightforward. We refer to a mutation as A1846T if A1846 is found in the early time point but T1846 is found at a later time point. For the cross-sectional study where a single serum sample is available, we refer to the sequence variant simply as A1846 or T1846, although sequences such as T1762, A1764, and A1896 are clearly mutants based on published longitudinal studies. We will define the wild-type and mutant sequences in other positions of the core promoter and preC/C region according to a recent large-scale study from China, where sequences of 846 asymptomatic HBsAg carriers infected with genotypes B and C were determined [Yin et al., 2011]. According to this study, A1846 and C1913 represent wild-type sequence, whereas T1846 and A/G1913 represent mutations.
Longitudinal Analysis of Mutations Accumulated During ACLF
Table I shows the liver functions, HBV DNA levels, genotypes, and HBeAg status of the six patients selected for longitudinal study. The four patients with ACLF (P1–P4) demonstrated a greater number of mutations (95, 49, 54, and 22, respectively) than the two patients with LC (P5 and P6; 14 and 21, respectively). Although mutations were found in all four open reading frames (ORFs), they were not distributed evenly. For P1–P4, mutations observed in two or more patients were C53T, A1846T, and G1896A (Table II). An unrooted phylogenetic neighbor-joining tree of all full-length HBV genomic sequences from six patients showed that virus from the same patient had the same origin but diverged subsequently (Fig. 1).
| Patient | Data of serum sample collection | Nucleotide position | HBeAg | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 53 | 1752 | 1753 | 1762 | 1764 | 1799 | 1846 | 1896 | 1899 | 1913 | 1915 | 2185 | |||
| P1 | 05/24/2005 | Ca | A | T | A | A | C | T | Ga | G | A | G | A | + |
| 12/16/2006 | T | A | T | A | A | C | Tb | A | G | Ab | G | A | − | |
| P2 | 07/03/2002 | T | A | T | T | A | C | Aa | G | G | Ca | A | A | + |
| 12/26/2005 | C | A | T | T | A | C | Ta | Aa | Ga | A | Aa | A | − | |
| P3 | 06/14/2005 | Ta | A | Ca | T | A | C | Aa | A | G | C | G | A | − |
| 08/20/2006 | Ta | A | C | T | A | C | Ta | A | G | C | G | A | − | |
| P4 | 08/16/2006 | C | A | T | A | G | C | T | A | A | C | G | A | − |
| 08/05/2008 | Ta | A | T | A | G | C | T | A | A | C | G | A | − | |
| P5 | 10/27/2003 | T | A | T | T | A | C | A | G | G | C | G | A | + |
| 12/19/2007 | T | A | T | T | A | C | A | G | G | C | G | G | + | |
| P6 | 04/19/2004 | C | A | T | T | A | C | A | A | G | C | G | A | − |
| 03/19/2007 | C | A | T | T | A | C | A | A | G | C | G | G | − | |
- a Found in two of three clones.
- b Found in three of four clones. Otherwise the same sequence was found in all the clones.
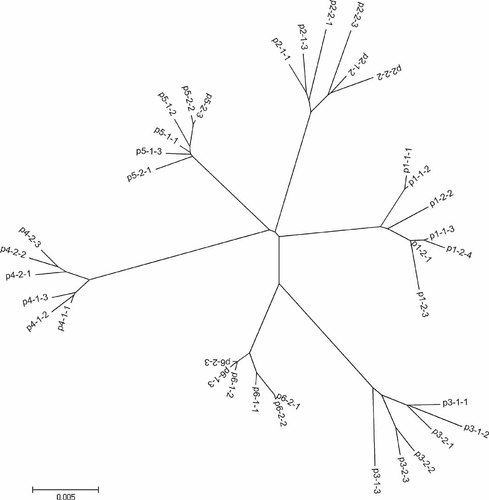
Unrooted phylogenetic tree of 37 full-length HBV genomic sequences obtained from six patients enrolled in the longitudinal study.
Cross-Sectional Analysis of BCP and PreC/C Mutations Among Four Groups of Patients
The baseline demographics, liver function tests, and virological data, which were obtained on the day of hospitalization, are listed in Table III. Among the 166 samples, 28 were infected with genotype B and 138 with genotype C. There were 91 HBeAg positive cases and 75 HBeAg negative cases (Table IV). The distribution of nucleotide changes in the BCP and preC/C region of 166 patients is outlined in Table V. Statistical analysis revealed significant differences in the prevalence of T1846 and A/G1913 between the ACLF and LC groups; and in the prevalence of T1762, T1846, and A/G1913 between the ACLF and HCC groups. These findings suggest that T1846 and A/G1913 are closely associated with ACLF.
| CHBa | LCb | ACLFc | HCCd | Pe | |
|---|---|---|---|---|---|
| Sex (M/F) | 36:9 | 34:11 | 42:7 | 21:6 | 0.753 |
| Age (years) | 33.9 ± 11.1 | 46.1 ± 10.4 | 45.8 ± 10.8 | 52.6 ± 10.5 | <0.001 |
| T.BIL (µmol/L) | 16.5 (6.2–79) | 43.3 (12–352) | 312.2 (172–641) | 63.9 (7.9–409) | <0.001 |
| ALT (U/L) | 132.9 (17–717) | 140.9 (16–752) | 396.7 (23–2633) | 73.7 (7–371) | <0.001 |
| PA (%) | 93.1 (65–124) | 62.6 (32–98.5) | 25.3 (0–39) | 67 (17.4–105) | <0.001 |
| HBV DNA (log value of copies/ml) | 6.91 ± 1.53 | 5.91 ± 1.24 | 4.88 ± 1.15 | 5.56 ± 1.51 | <0.001 |
| Genotype (B/C) | 12:32 | 5:40 | 9:40 | 2:25 | 0.101 |
| HBeAg (POS/NEG) | 36:9 | 20:25 | 23:26 | 12:15 | <0.001 |
- POS, positive; NEG, negative.
- Statistical analysis: e, among all groups; Age: a vs. b, c, d: P < 0.001; b vs. c: P = 0.999; b vs. d: P = 0.103; c vs. d: P = 0.074; T.BIL: a vs. b, c, d: P < 0.001; b vs. c: P < 0.001; b vs. d: P = 0.161; c vs. d: P < 0.001; ALT: a, b, d vs. c: P < 0.001; a vs. b, d: P > 0.05; b vs. d: P = 0.097; PA: a vs. b, c, d: P < 0.001; b, d vs. c: P < 0.001; b vs. d: P = 0.249; HBV DNA: a vs. b, c, d: P < 0.001; b vs. c: P < 0.001; b vs. d: P = 0.257; c vs. d: P = 0.021; HBeAg: a vs. b, c, d: P < 0.01; b vs. c, d: P > 0.05; c vs. d: P = 0.835.
| Nucleotide sequence [no. (%) of patients] | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N | G1752 | C/G1753 | T1762 | A1764 | G1799 | T1846 | A1896 | A1899 | A/G1913 | A/C1915 | |
| HBV genotype | |||||||||||
| B | 28 | 18 (64.3) | 1 (3.6) | 14 (50.0) | 14 (50.0) | 27 (96.4) | 16 (57.1) | 14 (50.0) | 3 (10.7) | 8 (28.6) | 1 (3.6) |
| C | 138 | 1 (0.7) | 45 (32.6) | 105 (76.1) | 110 (79.7) | 1 (0.7) | 42 (30.4) | 59 (42.8) | 20 (14.5) | 25 (18.1) | 29 (21.0) |
| P-value | <0.001 | 0.001 | 0.007 | 0.002 | <0.001 | 0.007 | 0.309 | 0.429 | 0.157 | 0.018 | |
| HBeAg status | |||||||||||
| POS | 91 | 12 (13.2) | 17 (18.7) | 64 (70.3) | 68 (74.7) | 14 (15.4) | 15 (16.5) | 10 (11.0) | 5 (5.5) | 12 (13.2) | 10 (11.0) |
| NEG | 75 | 7 (9.3) | 29 (38.7) | 55 (73.3) | 56 (74.7) | 14 (18.7) | 43 (57.3) | 63 (84.0) | 18 (24.0) | 21 (28.0) | 20 (26.7) |
| P-value | 0.300 | 0.004 | 0.401 | 0.567 | 0.361 | <0.001 | <0.001 | 0.001 | 0.014 | 0.008 | |
| Patients | Nucleotide site [no. (%) of patients] | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | G1752 | C/G1753 | T1762 | A1764 | T1766 | A1768 | T1773 | G1799 | T1846 | C1858 | A1896 | A1899 | A/G1913 | A/C1915 | |
| CHBa | 45 | 8 (17.8) | 4 (8.9) | 20 (44.4) | 19 (42.2) | 2 (4.4) | 2 (4.4) | 1 (2.2) | 12 (26.7) | 5 (11.1) | 1 (2.2) | 10 (22.2) | 1 (2.2) | 1 (2.2) | 5 (11.1) |
| LCb | 45 | 3 (6.7) | 13 (28.9) | 38 (84.4) | 39 (87.6) | 5 (11.1) | 1 (2.2) | 2 (4.4) | 5 (11.1) | 14 (31.1) | 0 (0) | 23 (51.1) | 7 (15.6) | 8 (17.8) | 8 (17.8) |
| ACLFc | 49 | 7 (14.3) | 15 (30.6) | 36 (73.5) | 40 (81.6) | 3 (8.4) | 3 (6.1) | 1 (2) | 9 (18.4) | 30 (61.2) | 0 (0) | 25 (51) | 9 (18.4) | 21 (42.9) | 10 (20.4) |
| HCCd | 27 | 1 (3.7) | 14 (51.9) | 25 (92.6) | 26 (96.3) | 2 (7.4) | 0 (0) | 1 (3.7) | 2 (7.4) | 9 (33.3) | 0 (0) | 15 (55.6) | 6 (22.2) | 3 (11.1) | 7 (25.9) |
| Pe-value | 0.189 | 0.001 | <0.001 | <0.001 | 0.705 | 0.525 | 0.893 | 0.114 | <0.001 | 0.439 | 0.007 | 0.054 | <0.001 | 0.427 | |
| Pf-value | — | 0.015 | <0.001 | <0.001 | — | — | — | 0.059 | 0.020 | — | 0.004 | 0.026 | 0.014 | — | |
| Pg-value | — | 0.009 | 0.004 | <0.001 | — | — | — | — | <0.001 | — | 0.004 | 0.011 | <0.001 | — | |
| Ph-value | — | <0.001 | <0.001 | <0.001 | — | — | — | 0.046 | 0.021 | — | 0.004 | 0.006 | — | — | |
| Pi-value | — | — | — | — | — | — | — | — | 0.003 | — | — | — | 0.009 | — | |
| Pj-value | — | 0.051 | — | — | — | — | — | — | — | — | — | — | — | — | |
| Pk-value | — | — | 0.045 | — | — | — | — | — | 0.020 | — | — | — | 0.004 | — | |
- e, among all groups; f, a vs. b; g, a vs. c; h, a vs. d; i, b vs. c; j, b vs. d; k, c vs. d.
Multivariable Analysis: Binomial Logistic Regression
Patients' age, frequency of T1753, A1762/G1764, T1846, A1896, A1899, and A/G1913, HBV genotype, and patients' HBeAg status were included in the multivariable model. The results suggested that T1846 and A/G1913 were independent factors for ACLF (Table VI), and odds ratios were 3.373 and 4.244, respectively.
| B | SE | Wald | Sig. | Exp(B) | |
|---|---|---|---|---|---|
| T1846 | 1.216 | 0.572 | 4.519 | 0.034 | 3.373 |
| A/G1913 | 1.446 | 0.575 | 6.313 | 0.012 | 4.244 |
| T1762/A1764 | −0.144 | 0.474 | 0.092 | 0.761 | 0.866 |
| C/G1753 | 0.129 | 0.481 | 0.073 | 0.788 | 1.138 |
| A1896 | −0.786 | 0.615 | 1.631 | 0.202 | 0.456 |
| A1899 | −0.113 | 0.567 | 0.040 | 0.842 | 0.893 |
| HBeAg | 0.124 | 0.587 | 0.045 | 0.832 | 1.132 |
| Genotype | 0.060 | 0.546 | 0.012 | 0.912 | 1.062 |
| Age | 0.030 | 0.019 | 2.618 | 0.106 | 1.030 |
| Constant | −2.019 | 1.208 | 2.790 | 0.095 | 0.133 |
DISCUSSION
In the present study, a longitudinal analysis was carried out to determine the relationship between ACLF and mutations in the entire HBV genome. Due to its low incidence but high mortality, longitudinal follow-up of ACLF patients is difficult. Secondly, most patients with CHB and LC are currently subject to anti-viral therapy with nucleos(t)ide analogs, which may complicate data interpretation. Lastly, due to the low viremia titers of patients with ACLF, amplification of the full-length genome by a single round of PCR may not be successful. Out of 21 samples from ACLF patients and matching samples from an earlier time point (>1 year interval), we were able to generate the full-length sequences of four patients before and during acute liver failure. Comparative sequence analysis revealed a nucleotide change at position 53 in patients P1, P2, and P4. This nucleotide encodes the Pre-S2 domain of envelope proteins and spacer region of the polymerase protein. The T53C mutation was reported to correlate with disease progression [Sung et al., 2008; Jiang et al., 2009], but two of the three patients with ACLF presented with the C53T rather than T53C mutation. Therefore, the significance of nt 53 mutation in ACLF remains unclear. All the four ACLF patients had T1846 and A1896. Furthermore, P2 and P3 showed A1846T change when ACLF was detected, while P1 and P2 showed G1896A change. In contrast, both P5 and P6 of the LC group showed A1846. This suggests that T1846 and A1896 may correlate with the severity of liver disease. The results of the longitudinal study was verified by a cross-sectional study involving 166 patients, which revealed higher prevalence of T1846 and A/G1913 in the ACLF group than the LC or HCC group. In this regard, the C1913A mutation was detected at the ACLF stage in patient P2 of the longitudinal study.
As an immunotolerant, HBeAg can remove HBe/HBcAg-specific Th cells [Milich and Liang, 2003; Tong, 2007]. Reduction or prevention of HBeAg production may contribute to weakened immune tolerance and increased immunopathogenesis. The G1896A mutation in the preC region abolishes HBeAg expression at the translational level. It is often present in patients with fulminant hepatitis [Bartholomeusz and Locarnini, 2001]. Such HBeAg negative HBV variants have also been linked to liver failure [Chen et al., 2004]. Mutations that modulate HBeAg expression at the transcriptional level occur at nt 1753, 1762, 1764, and 1766 in the BCP region [Buckwold et al., 1996; Li et al., 1999; Parekh et al., 2003; Chen et al., 2007; Qin et al., 2009]. In this study, we found that BCP and preC/C mutations of T1753G/C, A1846T, G1896A, G1899A, C1913A/G, and G1915A/C were more commonly found in HBeAg negative patients, although prevalence of HBeAg did not show a statistically significant difference among LC, ACLF, and HCC patients.
HBV genotype is another factor that influences the natural course of liver disease, especially in Asia where genotypes B and C are prevalent. Previous reports have indicated that patients of genotype C have more severe liver disease [Yuen et al., 2004]. Studies have also found that genotype C has a higher frequency of BCP mutations, which is thought to be a reason for the relationship between genotype C and a poor prognosis [Yuen et al., 2004]. In this study, we failed to observe a statistical difference in the ratios between genotypes B and C among four groups of patients, but the rate of T1846 was higher in genotype B than in genotype C.
The results of the longitudinal analysis suggested possible correlation of mutations at nt 1846 and 1896 with ACLF, while a cross-section analysis demonstrated association of T1846 and A/G1913 with ACLF. T1846 correlated with both genotype and HBeAg status, whereas A/G1913 correlated with HBeAg status alone. Therefore, binomial logistic regression analysis was used to further eliminate interfering factors. The results demonstrate that T1846 and A/G1913 were independent factors for ACLF, with odds ratios of 3.373 and 4.244, respectively. Therefore, patients with T1846 or A/G1913 have a three or fourfold higher risk of developing ACLF compared to those with A1846 and C1913.
How the A/G1913 mutation contributes to liver failure remains unclear. The C1913A/G mutation causes a substitution of the fifth amino acid in core protein from proline to threonine or alanine. This amino acid is located in the antigenic epitope of the HBcAg HLA II-restricting helper T cell [Pumpens and Grens, 2001]. Since a proline is usually found at the turns of protein secondary structure, loss of a proline may cause drastic structural change to the core protein. Bozkaya et al. [1996] conducted a longitudinal study and found that the prevalence of this change ranged from 0% to 25% in CHB patients and correlated with the progress of the disease. A similar finding was made very recently [Yin et al., 2011]. Our results showed that there were 49% ACLF patients with the A/G1913, which is much higher than the rates of CHB reported.
The wild-type sequence at position 1846 is T for most HBV genotypes, but A for genotypes B and C prevalent in China [Yin et al., 2011]. Earlier studies identified T1846 in patients with fulminant hepatitis [Laskus et al., 1995; McMillan et al., 1996]. Recently, Yin et al. [2011] found that A1846T mutation was associated with LC and HCC in genotype C infected patients, with the mutation rate lowest in asymptomatic carriers. Therefore, the A1846T mutation seems to be similar to core promoter mutations (T1753C, A1762T, G1764A, C1766T, T1768A) and G1896A precore mutation in association with age-dependent disease progression (LC and HCC) as well as with severe liver diseases (fulminant hepatitis and ACLF). The A1846T mutation is silent at the amino acid level. However, A1846 forms an extra translation initiation codon (ATG; positions 1846–1848) for a small ORF upstream of the core gene. This small ORF has a conserved ATG codon at positions 1852–1854, and a stop codon five nucleotides downstream of the core gene initiation codon (Fig. 2). Mutational analysis suggests that the small ORF negatively regulates core protein translation [Chen et al., 2005]. It is likely that translation initiation from ATG1852–1854 is inefficient because of its weak Kozak sequence. Presence of another ATG at 1846–1848 (which is located at the beginning of RNA secondary structure of the pregenome encapsidation signal) probably causes additional translation of this ORF which would further reduce core protein expression. In this scenario, the A1846T mutation can increase core protein expression, which probably increases genome replication and also triggers more vigorous immune clearance mechanism leading to liver failure. Direct transfection experiment is needed to verify this hypothesis.
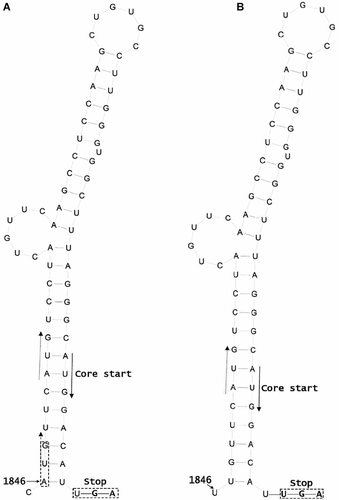
Possible influence of A1846T mutation on core protein translation. A: The wild-type sequence. The core gene translation initiation codon is located in a base-paired region of the pregenome encapsidation signal. It is preceded by a small ORF, which terminates just five nucleotides downstream and has been reported to down regulate core protein expression. A1846 generates an extra initiation codon (ATG) for this small ORF, which probably increases translation initiation of the small ORF and further reduces core protein translation. B: The mutant sequence. The A1846T mutation abolishes this extra ATG codon and possibly partially relieves the inhibitory effect of the small ORF on core protein translation.
Acknowledgements
We thank Dr. Panyong Mao of the Central Medical Lab of 302 Military Hospital for serum samples.




