Case report: A case of light chain deposition disease involving liver and stomach with chronic hepatitis C virus infection and hepatocellular carcinoma
Abstract
Light chain deposition disease (LCDD) is a rare, plasma cell proliferative disorder characterized by mainly abnormal light chain deposition in various organs. Hepatitis C virus (HCV) is a hepatotrophic and lymphotrophic virus and significantly related to B-cell proliferation. This is a case report of systemic LCDD involving liver, stomach, bone marrow, and probably kidney, in a patient with HCV-related hepatocellular carcinoma (HCC). A 62-year-old man with chronic HCV infection who presented with a small HCC in segment 8 of the liver and nephrotic syndrome showed kappa typed immunoglobulin light chain depositions in biopsy specimens of bone marrow, stomach, and non-tumorous liver parenchyma. After treatment of the HCC with transarterial chemoembolization, antiviral therapy for chronic hepatitis C was started. The patient showed early virologic response at 12 weeks of treatment; however, antiviral therapy was discontinued due to adverse effects and he was lost to follow-up. This is the first case of LCDD involving the liver and stomach in a patient with chronic HCV infection and HCC, which may represent LCDD as a rare HCV-associated B cell proliferative disease. J. Med. Virol. 83:810–814, 2011. © 2011 Wiley-Liss, Inc.
Introduction
Hepatitis C virus (HCV) infection is known to be related to lymphoproliferative diseases, such as cryoglobulinemia, B cell non-Hodgkin's lymphoma, and monoclonal gammopathy of undetermined significance [Lauer and Walker, 2001]. Light chain deposition disease (LCDD) is a systemic disease characterized by clonal proliferation of plasma cells, overproduction of abnormal light chain, and deposition of non-amyloid monoclonal immunoglobulin light chains in various organs, which could induce organ dysfunction, especially in the kidney [Buxbaum and Gallo, 1999; Pozzi et al., 2003]. Reports on monoclonal immunoglobulin deposition disease associated with chronic HCV infection have been rare [Miura et al., 2010]. A case is described of HCV-related HCC and cirrhosis of the liver complicated with systemic LCDD involving the stomach, liver, bone marrow, and probably kidney.
Case Presentation
Clinical History
A 62-year-old Mongolian man visited Seoul National University Bundang Hospital because of a liver mass detected on regular examination along with peripheral edema and foamy urine for the previous 2 months. He was healthy previously, with a history of chronic heavy alcohol drinking and a total of 13 pack-years as a current smoker. He denied blood transfusion or intravenous drug use in the past history. On physical examination, he was alert but showed a slightly puffy face. He was neither anemic nor icteric without palpable lymph nodes. Chest and abdomen showed no remarkable abnormality. His lower extremities showed pitting edema with erythematous skin lesions with some crusts. Vital signs included blood pressure of 148/96 mmHg, pulse rate of 53/min, respiratory rate of 18/min, and body temperature of 36.1°C.
The initial laboratory findings showed white blood cell count: 10,520/mm3; hemoglobin: 13.2 g/dL; platelet: 293,000/mm3; blood urea nitrogen: 36 mg/dL; creatinine: 1.7 mg/dL; uric acid: 10.1 mg/dL; cholesterol: 218 mg/dL; total protein: 6.1 g/dL; albumin: 2.0 g/dL; total bilirubin: 0.4 mg/dL; aspartate aminotransferase: 76 IU/L; alanine aminotransferase: 52 IU/L, and alkaline phosphatase: 276 IU/L. Urinalysis with microscopy revealed albuminuria of 3+ and a negative result for hematuria. The amount of urinary protein was 6.4 g/day. Cryoglobulin was negative, and serum immunoglobulin G (IgG) level was increased up to 2,580 mg/dL (normal range: 700–1,700 mg/dL). His initial serum level of beta 2-microglobulin was 13.29 mg/L (normal range: 1.0–2.4 mg/L), free kappa chain of 179 mg/L (3.3–19.4 mg/L), and free lambda chain of 93.3 mg/L (5.71–26.3 mg/L). He showed positive results for anti-HCV and HCV RNA with genotype Ib and serum RNA levels of 6,784,070 IU/ml. Hepatitis B surface (HBs) antigen was negative, anti-HBs positive, and anti-HIV negative. Serum levels of alpha-fetoprotein and protein induced by vitamin K absence were normal, 7.6 IU, and 26 AU/ml, respectively. Results for anti-nuclear antibody, anti-smooth muscle antibody, and anti-neutrophil cytoplasmic antibody were all negative with serum levels of complement 3 and complement 4 in the normal range (142 and 28 mg/dL, respectively). Both serum and urine protein electrophoreses documented monoclonal protein of 0.87 g/dL and 0.64 g/day, respectively. Monoclonal protein was revealed as IgG and kappa typed chains by immunofixation in serum.
A computed tomography (CT) and superparamagnetic iron oxide dynamic magnetic resonance image (MRI) of the liver revealed a solitary mass lesion measuring 1.8 cm in segment 8, which was a typical feature of hepatocelluar carcinoma (HCC) with arterial enhancement and delayed washout of contrast media (Fig. 1). Underlying liver showed cirrhotic features with mild ascites, and suspicious iron deposition in reticuloendothelial systems. Transarterial chemoembolization for the HCC lesion was performed with dense lipiodol uptake. Reasons for undertaking transarterial chemoembolization for treatment of HCC in this case included the presence of advanced cirrhosis, poor general condition, and no visualization of the target lesion on ultrasonography. No osteolytic lesion was observed on X-ray surveys for bone disease. Echocardiography and pulmonary function tests showed no remarkable abnormality.
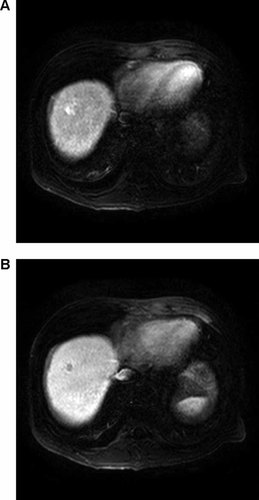
A solitary occupying lesion in S8 shows arterial enhancement (A) and delayed washout (B) of contrast media in liver MRI.
Bone marrow biopsy showed a normal trilineage of hematopoiesis with increase of plasma cells up to 12.5%. Microscopic findings of liver biopsy taken from non-tumorous liver parenchyma included mild inflammation in the periportal space and some plasma cell infiltration in hepatic tissue (Fig. 2) with characteristic amorphous Congo red-negative, eosinophilic material depositions without iron deposition (Fig. 2). Gastroendoscopic biopsy from antral erosions showed chronic gastritis with the same pattern of eosinophilic material deposition as that of the liver (Fig. 2). Immunohistochemical stains for kappa (polyclonal rabbit anti-human antibody, 1:50, Dako, Glostrup, Denmark) and lambda light chains (polyclonal rabbit anti-human antibody, 1:50, Dako) revealed that eosinophilic depositions in bone marrow, liver, and gastric mucosa were composed of kappa light chains (Fig. 2). Skin biopsy taken from the lower leg lesion showed papulosquamous dermatitis without lymphoid cell infiltration or active vasculitis.
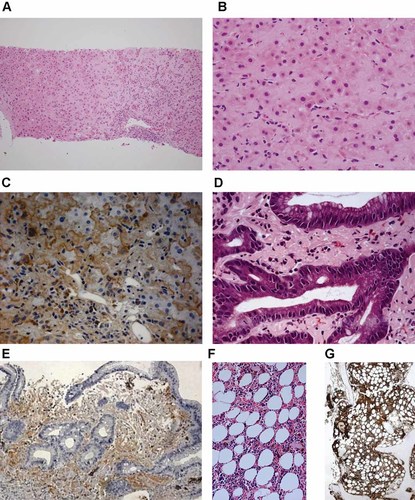
Histopathological findings. Needle biopsy of the liver demonstrates expansion of sinusoids with amorphous eosinophilic deposits and portal lymphoplasmacytic infiltration (A,B). Eosinophilic deposition is positive for kappa light chain by immunohistochemistry (C). Similar kappa light chain deposits are seen in the lamina propria of the gastric biopsy specimen (D,E) and bone marrow biopsy specimen (F,G) [Hematoxylin–eosin, original magnification ×40 (A), ×200 (B, D), ×100 (F); immunohistochemistry for kappa light chain, original magnification ×200 (C), ×100 (E), ×40 (G)]. [Color figure can be seen in the online version of this article, available at wileyonlinelibrary.com]
Clinical Course
The patient was diagnosed as multiple myeloma and systemic LCDD involving bone marrow, liver, stomach, and probably kidney with underlying chronic HCV infection and HCC. Hypertension with nephrotic syndrome was controlled by an angiotensin converting enzyme inhibitor. After transarterial chemoembolization for the small HCC, he was treated with Peg-IFNα-2b and a reduced dose of ribavirin to eradicate chronic HCV infection and possibly improve HCV-associated LCDD. At 4 weeks of antiviral therapy, HCV RNA was positive, but at 12 weeks of therapy, serum HCV RNA decreased to below 615 IU/ml, which suggested early virologic response without rapid virologic response. However, 24-hr urine protein level was slightly decreased from 6.1 g at pretreatment to 5.6 g at 12 weeks of treatment, while serum beta 2-microglobulin level increased from 13.3 to 22.2 mg/dL. During antiviral therapy, he became depressive with severe anorexia and deterioration of nutritional status, and his renal insufficiency was aggravated up to the creatinine level of 2.5 mg/dL. Antiviral therapy was discontinued, and he wanted to return to Mongolia. At 2 months after loss of follow-up, we heard about his death from his daughter; however, she did not know the cause of her father's death.
Discussion
This report describes a case of chronic HCV infection with HCC and multiple myeloma with LCDD, which involved the liver, stomach, bone marrow, and probably kidney and skin. Due to advanced cirrhosis, poor general condition, and no visualization on ultrasound, the patient was inappropriate for local treatment of HCC. After treatment of a small HCC using transarterial chemoembolization, antiviral therapy for chronic hepatitis C was tried with early virologic response, while LCDD did not show improvement. Antiviral therapy was discontinued and he died 2 months after loss to follow-up.
HCV is both a hepatotropic and lymphotropic virus; therefore, numerous extrahepatic manifestations related to B-cell proliferation are associated with HCV infection. Among them, mixed cryoglobulinemia, a prototype of B-cell lymphoproliferative disorder, has been the most closely associated with HCV infection. B-cell non-Hodgkin's lymphoma and monoclonal gammopathies of uncertain significance were also significantly associated with HCV infection. Regression of clonal proliferation in response to effective antiviral therapy has been observed in low-grade HCV-positive non-Hodgkin's lymphoma [Craxi et al., 2008].
LCDD is characterized by abnormal light chain deposition in various organs through clonal proliferation of plasma cells, which was associated with monoclonal gammopathies of uncertain significance in 17% and with multiple myeloma in 58% of cases [Lin et al., 2001]. The most commonly involved organ by LCDD is the kidney, and other organs, such as the brain, lung, heart, and liver can be involved [Piard et al., 1998; Toor et al., 2006; Popovic et al., 2007; Koopman et al., 2009; Nath et al., 2010]. However, LCDD involving the stomach has not been reported. Furthermore, the association between LCDD and HCV infection has rarely been reported in the literature. Miura et al. [2010] reported on a case of IgA lambda type deposition disease involving the kidney as a membranous nephritis in a patient with HCV infection who had been treated for 10 years before diagnosis of monoclonal immunoglobulin deposition disease; this patient underwent surgery for treatment of rectal cancer at 7 months after diagnosis of IgA lambda type deposition disease. Although several case series of LCDD involving the kidney have been reported [Lin et al., 2001], there have been no reports on LCDD involving the liver and stomach in a patient infected chronically with HCV.
Therefore, this is the first case report with documentation of light chain deposition in the liver, stomach, and bone marrow in a patient with chronic HCV infection and HCC, manifesting as nephrotic syndrome and skin lesions. Noticeable amorphous eosinophilic materials were documented in specimens of the gastric antrum and liver, which were unveiled as kappa light chain. With the existence of a monoclonal spike and increase of plasma cells in bone marrow, he was diagnosed initially as LCDD with multiple myeloma [Kyle and Rajkumar, 2009]. However, chronic HCV infection, coexistent HCC, and poor general condition led to transarterial chemoembolization and antiviral therapy, including pegylated interferon and low dose ribavirin for the first time, rather than systemic chemotherapy for treatment of myeloma. Renal biopsy was not performed due to the risk of bleeding, so that whether renal manifestations originated from LCDD was not elucidated, although his blood cryoglobulin was negative.
Interaction between HCV and lymphocytes may result in in vivo polyclonal activation and expansion of B cells. Clonal expansion of B cells occurs in the liver of almost 50% of HCV-infected patients, and, less frequently, in blood and bone marrow [Sansonno et al., 2009]. One study showed high prevalence of monoclonal gammopathies in patients with HCV infection compared to HCV negative patients [Pietro et al., 1998]. Direct infection of HCV to B cells has been reported by detection of HCV-minus-strand RNA (replicative intermediates) in lymphoid cells from HCV-infected patients with cryoglobulinemia [Inokuchi et al., 2009]. Infection of B cells with HCV may induce somatic mutations of over-expression of anti-apoptotic genes, or interaction between HCV and B cell signaling receptors may regulate survival of B cells. Several studies have reported that sustained antigenic stimulation is important to lymphomagenesis, as in H. pylori-induced marginal zone B-cell lymphoma arising in lymphoid tissues on gastric mucosae [Inokuchi et al., 2009]. This case of LCDD may represent a long-term result of chronic stimulation of B cells by HCV infection, which simultaneously plays a carcinogenic role in hepatocytes by different or interactive pathways.
In conclusion, this is the first case of LCDD involving the liver, stomach, and bone marrow in a patient infected chronically with HCV with a small hepatocellular carcinoma, which may represent the possibility of LCDD as a rare HCV-associated B cell proliferative disease. Further studies and case reports are needed.




