High activity of indoleamine 2,3-dioxygenase is associated with renal insufficiency in Puumala hantavirus induced nephropathia epidemica
Abstract
Nephropathia epidemica (NE) is a hemorrhagic fever with renal syndrome caused by Puumala hantavirus. The severity of NE varies greatly. The aim of the present study was to evaluate whether serum indoleamine 2,3-dioxygenase (IDO) activity is associated with the severity of NE. A prospectively collected cohort of 102 consecutive patients with acute serologically confirmed NE was examined. Serum kynurenine, tryptophan, creatinine, CRP, and blood cell count were measured for up to 5 consecutive days after admission. The kynurenine to tryptophan (kyn/trp) ratio reflecting IDO activity was calculated. A maximum kyn/trp ratio >202 µmol/mmol had a sensitivity of 85% and a specificity of 75% for detecting maximum serum creatinine values >250 µmol/L by receiver operating characteristic (ROC) analysis. A maximum kyn/trp ratio >202 µmol/mmol (high IDO level) was also associated with other parameters reflecting the severity of the disease and renal impairment. Patients with high IDO levels had higher maximum serum creatinine (379 vs. 102 µmol/L, P < 0.001), plasma C-reactive protein (104.1 vs. 72.1 mg/L, P = 0.029), and blood leukocyte values (11.9 vs. 9.0 × 109/L, P < 0.001) compared to patients with kyn/trp ratio ≤202 µmol/mmol. They also had lower minimum urinary output (1,100 vs. 1,900 ml/day, P < 0.001) and longer hospital stays (8 vs. 5 days, P < 0.001). In conclusion, high serum IDO activity was associated with increased disease severity and renal impairment in NE. J. Med. Virol. 83:731–737, 2011. © 2011 Wiley-Liss, Inc.
INTRODUCTION
Nephropathia epidemica (NE) is a mild type of hemorrhagic fever with renal syndrome (HFRS), caused by Puumala virus (PUUV) [Vapalahti et al., 2003]. The virus is a member of the Hantavirus genus in the Bunyaviridae family and is carried by bank voles (Myodes glareolus) [Vapalahti et al., 2003]. NE is prevalent in Finland, elsewhere in Scandinavia, in the Balkan region, and also in many parts of Western Europe [Kanerva et al., 1998; Vapalahti et al., 2003]. Approximately 1,000–3,000 serological diagnoses of PUUV infection are made in Finland annually (http://www3.ktl.fi/), and the seroprevalence in the population is 5% [Brummer-Korvenkontio et al., 1999]. Other hantaviruses causing HFRS include Hantaan, Dobrava, Saaremaa, and Seoul viruses; while in the Americas, Sin Nombre, Andes, and Black Creek Canal viruses cause hantavirus cardiopulmonary syndrome (HCPS) [Vapalahti et al., 2003].
The clinical severity of NE varies greatly and host genetics have been shown to influence the clinical picture [Mustonen et al., 1996; Mäkelä et al., 2002]. The usual symptoms are high fever, headache, backache, abdominal pain and nausea, while hemorrhagic manifestations are uncommon [Lähdevirta, 1971; Mustonen et al., 1994a; Settergren, 2000]. Renal involvement is manifested by proteinuria, hematuria, and oliguria followed by polyuria. A minority of patients require transient dialysis treatment [Lähdevirta, 1971; Mustonen et al., 1994a; Settergren, 2000]. The characteristic histopathologic renal finding is acute tubulointerstitial nephritis [Mustonen et al., 1994b]. Complete recovery is the usual outcome [Lähdevirta, 1971; Mustonen et al., 1994a; Settergren, 2000]. Typical laboratory findings in NE are leukocytosis, thrombocytopenia, anemia, and elevation of plasma C-reactive protein (CRP) and creatinine concentrations [Mustonen et al., 1994a; Settergren, 2000]. In addition radiological pulmonary manifestations have been detected in 16–53% of hospitalized patients with NE [Lähdevirta, 1971; Linderholm et al., 1992; Mustonen et al., 1994a; Kanerva et al., 1996; Paakkala et al., 2004].
Indoleamine 2,3-dioxygenase (IDO) is an enzyme catalyzing the first and rate-limiting step in the major pathway of tryptophan (trp) catabolism to kynurenine (kyn) and its derivatives [Mellor, 2005]. IDO is expressed widely in various immune cells, including monocyte-derived macrophages and dendritic cells [Mellor and Munn, 2004]. It is also expressed in other types of cells, such as certain tumor-cells, fibroblasts, and renal tubular epithelial cells (TEC) [Burke et al., 1995; Jalili et al., 2007; Mohib et al., 2007]. Interferon (IFN)-γ is the strongest inducer of IDO [Mellor and Munn, 2004]. Increased IDO activity results in the depletion of trp and this can lead to inhibition of T-cell responses and proliferation and thus to immunosuppression [Hwu et al., 2000; Mellor et al., 2002]. Depletion of trp is also thought to be the mechanism by which IDO activity inhibits the replication of various bacteria, intracellular parasites, and viruses [Takikawa, 2005].
In the present study, we investigated whether serum IDO concentrations are associated with disease severity and renal insufficiency in Puumala hantavirus-induced HFRS.
MATERIALS AND METHODS
Patients
The study cohort consisted of 102 prospectively identified consecutive patients with acute NE treated at Tampere University Hospital, Finland, between September 2000 and January 2008. The median patient age was 46 (range 22–77) years, and 69 (68%) were males.
Thirty-eight (37%) patients had one or more of the following diseases before NE: essential hypertension in 12, dyslipidemia in eight, arthritis rheumatoides and atrial fibrillation/fluctuation in four, and coronary artery disease and bronchial asthma in three patients; hypothyreosis, chronic inflammatory bowel disease, diabetes mellitus, psychiatric disorder, migraine and hyperplasia of the prostate in two patients; sick sinus syndrome treated with pacemaker, osteoporosis, psoriasis, Sjögren's syndrome, mitral valve disease, epilepsy, fibromyalgia, sarcoidosis, multiple sclerosis, polyneuropathia, chronic gastritis, transient ischaemic attack, congenital hearing impairment, operated atrial septal defect, and operated melanoma in one patient each.
The diagnosis of acute PUUV infection was serologically confirmed in all cases [Vapalahti et al., 1996]. All subjects gave informed consent before participation and the study was approved by the Ethics Committee of Tampere University Hospital and the University of Massachusetts Medical School.
Study Protocol
All 102 patients were studied during the acute phase of NE. A detailed past and current medical history was obtained, and a careful physical examination was performed. Blood samples to analyze serum trp and kyn, creatinine, CRP, and blood cell count were collected for up to 5 consecutive days after hospitalization. Other blood samples were taken according to the clinical needs of the patient. The highest and the lowest value of each patient of the various variables measured during hospitalization were designated as the maximum and minimum values. In this study, we have defined significant renal insufficiency as a serum creatinine value exceeding 250 µmol/L. Minimum urinary output was defined as low if it was equal or lower than the median value in the study population (≤1,440 ml/day) and high if it was >1,440 ml/day.
METHODS
All blood specimens were obtained between 7:30 and 9:30 in the morning. Plasma CRP was analyzed by the Roche Diagnostics CRP method using Cobas Integra analyzer (F. Hoffman-La Roche Ltd, Basel, Switzerland). Blood cell count was completed by hematological cell counters by Bayer. Serum creatinine was determined by Cobas Integra analyzer.
The rate of trp degradation reflects the IDO enzyme activity, and IDO level can thus be measured by determining the ratio of kynurenine to tryptophan (kyn/trp) in sera [Schrocksnadel et al., 2006]. Serum trp and kyn concentrations were measured afterwards from frozen samples by reverse-phase high-performance liquid chromatography (HPLC), as previously described [Laich et al., 2002]. Trp was separated with a Shimadzu liquid chromatograph LC-10AD VP (Shimadzu Co, Kyoto, Japan) using a 50-mm BDS Hypersil C18 5 µm column (Thermo Electron Co, Bellefonte, PA). It was monitored by fluorescence with a Shimadzu RF-10A XL detector at 266 nm excitation and 366 nm emission wavelengths. Kyn was separated with a Hewlett Packard 1100 liquid chromatograph (Palo Alto, CA) using a Merck LiChroCart 55–4,150 mm cartridge containing a Purospher STAR RP-18 3 µm column (Merck Co, Darmstadt, Germany). It was determined by ultraviolet absorption at 360-nm wavelength with a Hewlett Packard G13144 detector. The kyn/trp ratio was calculated by relating concentrations of kyn to trp, this allowing estimation of IDO activity.
Statistical Analysis
In order to describe the data, medians and ranges were given for continuous variables and numbers and percentages for categorical variables. To evaluate the associations of plasma IDO levels with the severity of NE, we divided the patients into two groups according to the maximum IDO value. Groups were compared using the Mann–Whitney U-test. Categorical data were analyzed by the χ2 test or the Fisher's exact test. Correlations were calculated by means of Spearman's rank correlation coefficient. Wilcoxon's test was used to compare two related samples. We also performed a logistic regression analysis with significant renal insufficiency (creatinine >250 µmol/L) as a dependent factor and high IDO and low urinary output as independent factors in order to further examine the associations of these factors with significant renal insufficiency. Age and sex were also included in these models. Odds ratios (OR) and their 95% confidence intervals (95% CI) were given. All tests were two-sided, and statistically significant P values are given. All analyses were made with the SPSS (version 7.5) statistical software package.
RESULTS
The clinical and laboratory characteristics of the NE patients are shown in Table I. The median duration of symptoms before admission to the hospital was 4 days (range 1–15 days). None of the patients was in clinical shock at the time of admission. Five of the 102 NE patients (5%) eventually needed dialysis treatment during hospital stay. The median duration of fever was six days (range 2–19 days). Everyone recovered completely.
| Median | Range | |
|---|---|---|
| BMI (kg/m2) (n = 83) | 26.2 | 18.5–37.0 |
| Urinary output min (ml/day) (n = 94) | 1,440 | 50–4,940 |
| Change in weight (kg) | 2.1 | 0–12.0 |
| Hospital stay (days) | 6 | 2–15 |
| IDO max (µmol/mmol) | 199.3 | 46.6–3,679.2 |
| Creatinine max (µmol/L) | 176 | 52–1,285 |
| CRP max (mg/L) | 79.8 | 15.9–269.2 |
| Leukocytes max (109/L) | 10.1 | 3.9–31.2 |
| Hematocrit min | 0.36 | 0.25–0.44 |
| Platelets min (109/L) | 61 | 9–238 |
- BMI, body mass index; min, minimum; max, maximum; IDO, serum kynurenine/tryptophan ratio; CRP, plasma C-reactive protein.
The ability of maximum serum kyn/trp ratio to predict serum creatinine levels >250 µmol/L was evaluated using receiver operating characteristics (ROC) curves [Boyd, 1997]. A maximum kyn/trp >202 µmol/mmol showed a sensitivity of 85% and a specificity of 75% for detecting maximum serum creatinine levels >250 µmol/L and the area under curve (AUC) was 0.84 (95% CI 0.76–0.91; Fig. 1). This cut-off point was used to divide patients into two groups. Patients with low IDO value had maximum kyn/trp ratio ≤202 µmol/mmol and patients with high IDO value had maximum kyn/trp ratio >202 µmol/mmol. IDO values during acute illness and hospitalization were significantly higher (median maximum kyn/trp 199.3, range 46.6–3,679.2 µmol/mmol) than convalescent values taken 14–32 days (median 22 days) after the onset of fever (median 64.7, range 23.9–350.6 µmol/mmol, P < 0.001).
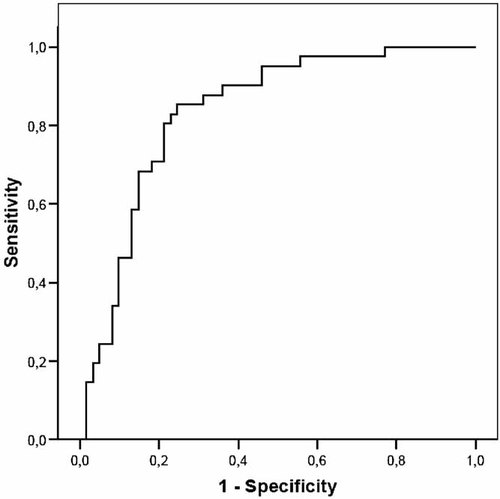
Receiver operating characteristics (ROC) curve for maximum kynurenine/tryptophan ratio in relation to serum creatinine value >250 µmol/L in 102 patients with nephropathia epidemica.
The proportion of males and females did not differ between patients with high serum IDO levels and patients with low IDO levels (70% male vs. 65% male, high vs. low IDO groups, respectively, P = 0.618). Forty six (45%) of the patients were smokers. The proportion of smokers also did not differ between high and low IDO groups (52% smokers vs. 44% smokers, high vs. low IDO groups, P = 0.404). All five patients, who needed dialysis treatment were in the high IDO group (P = 0.025). The maximum level of IDO was associated with several other parameters reflecting disease severity and renal impairment (Table II). Patients with high IDO levels were older than patients with low IDO levels, while BMI did not differ between these two groups. There was a strong positive correlation between maximum serum IDO and creatinine levels (r = 0.672, P < 0.001), as well as maximum IDO level and change in weight, which reflects fluid retention during renal impairment (r = 0.526, P < 0.001). Maximum IDO levels and minimum urinary output were inversely correlated (r = −0.385, P < 0.001). There was a positive correlation between maximum IDO levels and the length of hospital stay (r = 0.494, P < 0.001). Maximum IDO levels and the maximum blood leukocyte count were positively correlated (r = 0.508, P < 0.001). We also performed a logistic regression analysis in order to evaluate the association of high IDO levels with significant renal insufficiency (creatinine > 250 µmol/L) when adjusted for age, sex, and low urinary output (Table III). A high IDO level was an independent risk factor for significant renal insufficiency in this model.
| IDO max ≤202 µmol/mmol | IDO max >202 µmol/mmol | P | |
|---|---|---|---|
| n = 52 | n = 50 | ||
| Age (years) | 38 (22–77) | 50 (25–74) | 0.002 |
| BMI (kg/m2) (n = 83) | 24.8 (18.5–34.6) | 27.1 (20.9–37.0) | 0.149 |
| Urinary output min (ml/day) (n = 94) | 1,900 (200–4,940) | 1,100 (50–4,900) | <0.001 |
| Change in weight (kg) | 1.0 (0–10.0) | 3.5 (0–12.0) | <0.001 |
| Hospital stay (days) | 5 (2–15) | 8 (3–14) | <0.001 |
| Creatinine max (µmol/L) | 102 (52–537) | 379 (75–1,285) | <0.001 |
| CRP max (mg/L) | 72.1 (15.9–176.0) | 104.1 (19.7–269.2) | 0.029 |
| Leukocytes max (109/L) | 9.0 (3.9–24.0) | 11.9 (6.3–31.2) | <0.001 |
| Hematocrit min | 0.38 (0.31–0.44) | 0.33 (0.25–0.40) | <0.001 |
| Platelets min (109/L) | 53 (14–172) | 67 (9–238) | 0.202 |
- Values are expressed as medians (range). BMI, body mass index; min, minimum; max, maximum; IDO, serum kynurenine/tryptophan ratio; CRP, plasma C-reactive protein.
| Creatinine ≤250 µmol/L, N = 53 | Creatinine >250 µmol/L, N = 41 | OR | 95% CI | |
|---|---|---|---|---|
| Age (years) | 43 | 48 | 0.98 | 0.94–1.03 |
| Sex | ||||
| Female | 18 | 9 | 1 | Reference |
| Male | 35 | 32 | 2.70 | 0.80–9.09 |
| Low urinary output | ||||
| No | 33 | 14 | 1 | Reference |
| Yes | 20 | 27 | 1.96 | 0.65–5.87 |
| High IDO | ||||
| No | 39 | 6 | 1 | Reference |
| Yes | 14 | 35 | 17.57 | 5.25–58.77 |
- Continuous variables are expressed as medians. Category variables are expressed as numbers. IDO, kynurenine/tryptophan ratio; OR, odds ratio. CI, confidence interval.
We also analyzed maximum kyn and trp values separately in relation to parameters reflecting the severity of NE. Maximum kyn level was positively correlated with maximum serum creatinine (r = 0.785, P < 0.001) and change in weight (r = 0.517, P < 0.001). It was inversely correlated with minimum urinary output (r = −0.357, P < 0.001). There was also a positive correlation between maximum kyn level and the length of hospital stay (r = 0.517, P < 0.001) and the maximum blood leukocyte value (r = 0.516, P < 0.001). Maximum trp level had a slight positive correlation with minimum urinary output (r = 0.230, P = 0.026), and a slight inverse correlation with change in weight (r = −0.201, P = 0.044), the maximum blood leukocyte value (r = −0.244, P = 0.014) and maximum CRP (r = −0.256, P = 0.009).
As the patients with NE sought medical assistance at different time intervals from the onset of fever, the serum trp and kyn samples were also taken at different periods from the onset. From the majority of patients (80%) we had trp and kyn samples taken 6 days from the onset of fever. The median kyn/trp level on that day was 191.9, range 40.9–2,442.5 µmol/mmol. In this subgroup of 82 patients, the main results remained the same. Patients with high IDO (kyn/trp >202 µmol/mmol) 6 days after the onset had higher maximum blood leukocytes (median 12.3, range 6.3–31.2 × 109/L vs. median 9.1, range 5.1–24.0 × 109/L, P = 0.001), higher maximum serum creatinine (median 445, range 79–1,285 µmol/L vs. median 113 g range 60–615 µmol/L, P < 0.001), greater change in weight (median 3.5, range 0–12.0 kg vs. median 1.6, range 0–9.9 kg, P < 0.001), and longer hospital stay (median 7, range 3–14 days vs. median 5, range 2–10 days, P < 0.001) than patients with low IDO. They also had lower minimum urinary output (median 1,085, range 50–4,900 ml vs. median 1,555, range 200–4,940 ml, P = 0.042) than patients with low IDO.
In order to analyze the kinetics of the changes in serum IDO and creatinine levels, we depicted their daily medians in relation to the day of the onset of fever. Figure 2 shows that both variables first gradually increased to their peak values, and thereafter they started to decline. Median IDO peaked to 243.9 µmol/mmol 9 days after the onset of fever, whereas median creatinine was at its highest (265 µmol/L) 10 days after the onset of fever.
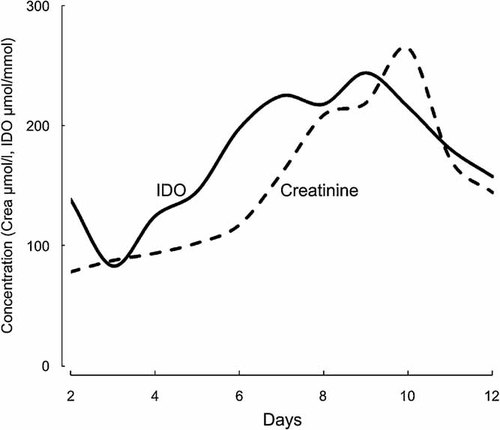
Daily median serum kynurenine/tryptophan (kyn/trp) ratios and creatinine levels in relation to the onset of fever. Kyn/trp median is presented as solid line and creatinine median as dashed line. IDO, serum kynurenine/tryptophan ratio; crea, serum creatinine.
DISCUSSION
The present study with 102 consecutive and prospectively identified hospitalized patients is to our knowledge the first study on IDO during a hantavirus infection. Our data show that IDO levels are elevated during acute Puumala hantavirus infection. The median maximum kyn/trp ratio in our study was 199.3 µmol/mmol during acute NE. Previous studies on IDO levels in patients with systemic lupus erythematosus (SLE) and in bacteremic patients have been carried out in Tampere University Hospital using the same laboratory as in the present study. In SLE patients the median IDO levels were 39.1–43.0 µmol/mmol during different seasons [Pertovaara et al., 2007]. In the same study the median kyn/trp ratio was also analyzed in 309 healthy controls and was found to be 25.9 µmol/mmol. Interestingly, in bacteremic patients, the median maximum kyn/trp ratio was lower (89.9 µmol/mmol) than in our patients with Puumala virus infection (199.3 µmol/mmol) [Huttunen et al., 2010]. In the present study, the IDO levels were lower during the convalescent phase (14–32 days after the onset of fever) than during the acute phase, but still higher than the values published earlier for the healthy controls. It was not possible to standardize dietary intake of the essential amino acid trp in our study. However, it seems unlikely that dietary intake would have affected serum kyn/trp levels, since kyn/trp ratio is not affected by the dietary intake of trp [Schrocksnadel et al., 2006].
In the present study, high IDO levels were associated with increased disease severity, especially renal impairment in acute Puumala hantavirus infection. A high IDO level was found to be an independent risk factor for maximum serum creatinine exceeding 250 µmol/L, when examined together with low urinary output, age, and sex. We found that high IDO levels were associated with significant renal insufficiency and higher blood leukocyte count as well as with lower urinary output and blood hematocrit. It also associated strongly with the duration of hospitalization.
IDO has not been widely studied in patients with viral infections. Increased IDO activity has been found in acute dengue virus infection and chronic hepatitis C virus infection [Larrea et al., 2007; Becerra et al., 2009]. Also increased trp degradation has been found in chronic Epstein–Barr virus infection [Bellmann-Weiler et al., 2008]. In those studies the association between IDO level and the severity or progression of the disease was not studied. However, in human immunodeficiency virus infection enhanced trp degradation by IDO was associated with disease progression and complications, for example, weight loss and neuropsychiatric disorders [Schroecksnadel et al., 2007]. High IDO levels were also associated with severe disease and mortality in bacteremic patients [Huttunen et al., 2010]. Treatment with granulocyte-macrophage colony-stimulating factor resulted in reduced IDO levels in severe sepsis, possibly due to improved antibacterial defence [Schefold et al., 2010]. In the present study, a high IDO level was associated with increased severity of Puumala hantavirus infection.
Studies of IDO in humans with kidney diseases are also scarce. It has been demonstrated that concentrations of trp metabolites increase in chronic renal insufficiency [Saito et al., 2000; Schefold et al., 2009]. This is presumed due to an increase in production and/or decrease in degradation, rather than a decrease in renal excretion [Saito et al., 2000]. In our study, the level of trp metabolite, kyn, positively correlated with the severity of acute renal insufficiency. This may suggest that the increased production of kyn reflecting the activity of IDO is important. In kidney allograft recipients, upregulation of IDO is associated with rejection [Brandacher et al., 2007]. In mice, it has been shown that IDO promotes renal ischemia-reperfusion injury [Mohib et al., 2008]. On the other hand, in nephrotoxic serum nephritis, a model of crescentic glomerulonephritis, IDO acts as a protective factor [Hou et al., 2009].
The pathogenesis of NE is not well understood. PUUV has no direct cytopathic effect on cultured cells [Temonen et al., 1993]. Therefore, it is unlikely that direct viral cytotoxicity is the primary cause of pathology in NE. PUUV causes acute tubulointerstitial nephritis and the severity of histological changes in NE has been found to be related to the clinical severity of renal failure as measured by the highest serum creatinine level [Mustonen et al., 1994b]. In kidney biopsies of patients with NE, there is an increased amount of infiltrating cells in the peritubular areas [Temonen et al., 1996]. The infiltrating cells include plasma cells, monocytes/macrophages, and lymphocytes, as well as polymorphonuclear cells, mainly eosinophils and neutrophils. CD8+ T-cells predominate the lymphocyte infiltrate [Temonen et al., 1996]. This is thought to indicate that cell-mediated cytotoxicity is important in the kidney involvement in NE.
Macrophages express IDO when exposed to IFN-γ. Increased IDO activity in turn results in T-cell supression and the induction of T-regulatory cells (T-regs). By a positive feedback loop, T-regs can further enhance IDO activity by stimulating increased IFN-γ expression [Mulley and Nikolic-Paterson, 2008]. It has been demonstrated that increased IDO activity promotes TEC apoptosis and inhibition of IDO augments TEC survival [Mohib et al., 2007]. We found that high IDO was strongly associated with significant renal failure. We also found that IDO levels peaked before creatinine levels, which might suggest that IDO could be in fact involved in the pathogenesis of renal failure in NE. One pathogenetic mechanism could be TEC apoptosis. Previously signs of epithelial cell apoptosis have been detected in patients with PUUV infection [Klingström et al., 2006]. Increased IDO levels could also contribute to immunosuppression through T-cell suppression and apoptosis.
In conclusion, a high serum kyn/trp level reflecting increased IDO activity was associated with clinically severe PUUV-induced interstitial nephritis and we suggest that it might even be involved in the pathogenesis of renal failure in NE.
Acknowledgements
The skilful technical assistance of Ms Katriina Yli-Nikkilä and Ms Mirja Ikonen is greatly appreciated.




