CASP8 promoter polymorphism is associated with high-risk HPV types and abnormal cytology but not with cervical cancer†
The authors declare no conflict of interest.
Abstract
Only a small fraction of women infected with human papillomavirus (HPV) progress to cervical cancer pointing to additional risk factors including host genetics that might play a role in development of cervical cancer. Caspase-8 (encoded by CASP8 gene) is crucial in generating cell death signals to eliminate potentially malignant cells. Genetic variation in CASP8 might influence the susceptibility to cancer. CASP8 -652 6N ins/del polymorphism has been previously reported to influence the progression to several cancers including cervical cancer. This polymorphism was investigated in 445 women of black African and Mixed Ancestry origin with invasive cervical cancer and 1,221 controls matched (1:3) by age, ethnicity, and domicile status. Genotyping for CASP8 -652 6N ins/del was done by PCR-RFLP. In the control women cervical disease was detected by cervical cytology. The CASP8 -652 6N del/del genotype did not show any significant association (P = 0.948) with cervical cancer. Further analysis within the controls showed a weak association (P = 0.048) of this polymorphism with abnormal cytology in both ethnicities and high-risk HPV infection (P = 0.030) only in the black Africans. This is the first study of the role of CASP8 -652 6N ins/del polymorphism in cervical cancer in an African population. These results show that CASP8 -652 6N del/del genotype increases the risk of abnormal cytology and high-risk HPV infection but does not show an association with cervical cancer. This result points towards an important role of CASP8 in HPV infection and in the development of pre-cancers. J. Med. Virol. 83:630–636, 2011. © 2011 Wiley-Liss, Inc.
INTRODUCTION
Cervical cancer is the second most frequent cancer and the highest cause of cancer related deaths in women worldwide. Approximately 493,243 new cervical cancer cases and 273,505 cervical cancer deaths are reported per year [Castellsague et al., 2007]. In sub-Saharan Africa an estimated number of over 70,000 new cases of cervical cancer occur each year which accounts for approximately 25% of all cancers becoming the most common cancer and the highest cause of cancer mortality in women [Parkin et al., 2008]. Due to poor screening programs and follow-ups more than 80% of all new cervical cancer cases and more than 85% of all cervical cancer-related deaths now occur in developing countries [Anorlu, 2008].
Cervical cancer develops slowly for several years through a multi-step process due to persistent infection of the epithelial cells with human papillomavirus (HPV). Oncogenic HPVs are the cause of more than 99% of all cervical cancer cases. High-risk HPVs such as type 16, 18, 31, 33, and 45 are associated with up to 97% of all cervical cancer cases worldwide [Walboomers et al., 1999; Clifford et al., 2003]. Persistent infection by oncogenic HPV types leads to squamous intraepithelial lesions (SIL) [Rohan et al., 2003]. SIL are further divided into low-grade SIL (LSIL) and high-grade squamous intraepithelial lesions (HSIL) lesions. Approximately 60% of the LSIL and 83% of the individuals with HSIL have been shown to be HPV infected [Allan et al., 2006]. LSILs are pre-cancerous (i.e., are very early precursor stages of cervical cancer) with very few cases progressing to cancer [Snijders et al., 2006]. On the other hand, most HSILs progress to invasive cervical cancer (ICC), the final stage of the cancer of the cervix if left untreated [Kobayashi et al., 2004].
Mucosal HPV infection constitutes one of the most common sexually transmitted viruses worldwide. Up to 80% of women around the world are infected with HPV by the age of 50 [Myers et al., 2000], although most of them are able to clear the virus. Less than 1% of the clinically detectable HPV is associated with progression to cervical cancer [Tindle, 2002] indicating an important role for other additional risk factors in the development of the cancer of the cervix. These risk factors include host and viral genetic factors, environmental, and life style factors [Martin et al., 2007], most of which likely influences response to HPV infection and its persistence. The role of host genes associated with cell-mediated immunity is supported by the involvement of macrophages, natural killer cells and T-cells in regressing papilloma in the risk of infection and persistence of HPV [Martin et al., 2007].
Apoptosis, a form of programmed cell death, is an important aspect of the human immune system, used to control tumors by eliminating potentially malignant cells in order to maintain tissue homeostasis [Evan and Vousden, 2001]. Thus reduced apoptosis is associated with progression to and formation of cancer [Lowe and Lin, 2000]. One of the main characteristics of cancer is to bypass apoptosis by forcing the cell to ignore signals that under normal circumstances would lead to cell suicide [Hanahan and Weinberg, 2000]. Signals generated by the interaction of cell surface death receptor Fas together with Fas ligand (FasL) activates caspase leading to apoptosis [Suda et al., 1993; Siegel, 2006]. The expression levels and activities of caspase enzymes have been shown by several studies as some of the most important mechanisms for regulating apoptosis and have also been associated with many pathological conditions including cancer [Siegel, 2006]. Mammalian cells undergo apoptosis through two principle pathways, i.e., the receptor (extrinsic) and the mitochondrial (intrinsic) pathway [Fulda and Debatin, 2006]. Stimulation of any of these pathways can lead to the activation of caspases [Degterev et al., 2003]. Various caspases are found downstream of Fas and FasL, among which caspase-8 plays one of the most important roles in generating apoptotic signals [Siegel, 2006]. It is central in activating a caspase-cascade receiving apoptotic signals from Fas–FasL interaction which triggers apoptosis [Ju et al., 1995]. However, besides apoptosis caspase-8 is involved in other process including a role in cell proliferation and differentiation [Geciras-Schimnich et al., 2002; Launay et al., 2005; Koenig et al., 2008]. Caspase-8 is encoded by a gene called, CASP8 which is sometimes referred to as FLICE or MCH5 and is localized on chromosome 2q33. Polymorphic variations in CASP8 gene might result in differential expression of caspase-8 leading to altered apoptosis and eventually influence the tumor progression and susceptibility to cancer.
Two polymorphisms in the CASP8 gene have been well studied, namely CASP8 D302H and CASP8 -652 6N ins/del [Sergentanis and Economopoulos, 2009]. Among these only CASP8 -652 6N ins/del (rs3834129) polymorphism has been associated with susceptibility to cervical cancer [Sun et al., 2007]. The CASP8 -652 6N ins/del polymorphism is a result of six nucleotide deletion of AGTAAG at position 652 in the promoter region of the CASP8 gene. This six nucleotide deletion destroys a binding element for transcriptional activator stimulatory protein-1 (SP1) which decreases capase 8 expression leading to reduced caspase-8 levels thereby affecting downstream processes including apoptosis [Sun et al., 2007]. There are conflicting reports on the role of CASP8 -652 6N ins/del polymorphism with respect to risk for pathological conditions such as cancer [Sun et al., 2007; Cybulski et al., 2008; Frank et al., 2008; Li et al., 2008; Pittman et al., 2008; Yang et al., 2008; De et al., 2009; Ji et al., 2009; Ni et al., 2009; Wang et al., 2009]. A study among Chinese subjects [Sun et al., 2007] reported an association of the CASP8 -652 6N del/del genotype with reduced risk of lung, oesophageal, gastric, breast, and colorectal cancer. In addition, another study among American Caucasians [Li et al., 2008] showed that the CASP8 -652 6N del variant was associated with reduced risk of cutaneous melanoma. The above findings of a role for the CASP8 -652 6N del/del genotype have been supported by reports of this genotype being associated with decreased sperm apoptosis and poor sperm motility [Ji et al., 2009] and with increased risk of Coal Worker's Pneumoconiosis [Ni et al., 2009] in Chinese population. However, studies among German [Frank et al., 2008], Polish [Cybulski et al., 2008] and Italian [De et al., 2009] populations do not support for the CASP8 -652 6N del variant with breast and prostate [Cybulski et al., 2008] cancer risk but instead the variant has been associated with later age at diagnosis for breast cancer in the Italian population [De et al., 2009]. Furthermore, the CASP8 -652 del variant was not associated with risk for colorectal cancer in the British population [Pittman et al., 2008].
Most of the research on host genetic susceptibility to cervical cancer on cell death pathway genes has focused on genes encoding Fas and FasL. To the best of our knowledge, only one study investigated the influence of CASP8 polymorphism on development of cervical cancer [Sun et al., 2007]. Sun et al. [2007] studied the role of CASP8 -652 6N ins/del polymorphism in a Chinese population and reported a reduced risk of developing cervical cancer with CASP8 -652 6N del/del [Sun et al., 2007].
No studies have been done in African populations on this polymorphism. Therefore, the aim of this study was to investigate the role of CASP8 -652 6N ins/del polymorphism in risk of cancer of the cervix in two groups of South African women, black Africans, and subjects of Mixed Ancestry origin.
MATERIALS AND METHODS
Study Participants
DNA from a total of 1,666 subjects comprising 445 women with ICC (106 black African and 339 women of Mixed Ancestry) and 1,221 controls (257 black African and 964 women of Mixed Ancestry) without cancer of the cervix was studied. Incident cases of symptomatic invasive cervical epithelial cancer (stage 1b-IVb), diagnosed a maximum of 6 months previously were recruited from Groote Schuur and Tygerberg Hospitals in the Western Cape Province, South Africa. Hospital controls were series matched in a ratio of 3:1 to the cases on decade of age, ethnic group, and area of residence (urban/rural). Among the cases 59% came from the urban area and 41% were of rural origin, while among the controls, 53% lived in the urban and 47% stayed in the rural area. The cases and controls formed part of a study to investigate the association of oral contraceptives with cervical cancer [Hoffman et al., 2003; Shapiro et al., 2003].
The mean age for black cases was 43.8 years (SD 9.2) and for Mixed Ancestry cases it was 45.9 years (SD 8.1). The mean age for black controls was 42.3 years (SD 9.1) and for Mixed Ancestry controls it was 44.3 years (SD 8.4). The HIV infection status was 5% for the cases and 4.7% for the controls. No significant differences in age or HIV status were observed between cases and controls (data not shown here). Among 1,230 controls for which pap smear results were available, 15% (n = 180) were positive for atypical squamous cells of undetermined significance and LSILs (n = 87) and HSILs (n = 43) while 85% (n = 1,050) were negative for pap smear. There was a significant difference in the smoking status between cases and controls (P = 0.001, OR (95% CI) = 1.60 (1.23–2.07), adjusted for ethnicity) (data not shown here). Subsequently, all the analyses were adjusted for the smoking status along with ethnicity. The observed genotype frequencies for CASP8 -652 6N ins/del polymorphism were found to be in Hardy–Weinberg equilibrium (HWE) for the black controls (P = 0.614) but slightly out of HWE in the Mixed Ancestry controls (P = 0.045).
Clinical Specimens
Following written informed consent blood was collected from cases and controls, and stored at −80°C. The study was approved by the University of Cape Town Human Research Ethics Committee (REC REF: 075/2009). The samples were completely blinded for this part of the investigation.
Papanicolaou Test
Papanicolaou tests (Pap smear) were conducted on endocervical scrapings taken from the control women as described previously [Shapiro et al., 2003]. For this paper, Pap smears with the cytological diagnosis of atypical squamous cells of undetermined significance, LSILs, or HSILs were considered as abnormal cytology.
High-Risk HPV Type Detection
Endocervical scrapings from control women were assayed for HPV infection using the Hybrid Capture II HPV Test for the detection of high risk HPV types 16/18/31/33/35/39/45/51/52/56/58/59/68, and classified as positive according to the manufacturer's instructions (Digene Corporation, Gaithersburg, MD) as described earlier [Shapiro et al., 2003]. 17% (208) of the total controls (1,229) for which a Hybrid Capture test was done were positive and 83% (1,089) were negative.
Extraction of Genomic DNA
The genomic DNA was extracted using TotalNucleicAcid Extraction kit for MagNA Pure Compact nucleic acid extractor (Roche Diagnostics, Germany).
Determination of CASP8 -652 6N ins/del Polymorphism
The CASP8 -652 6N ins/del polymorphism (rs3834129:–/AGTAAG; NM_001228.4) was determined using PCR/RFLP as described by Sun et al. [2007]. PCR was carried in a The PCR was carried in a total volume of and the reaction consisted of the primer pair; 5′CTGCATGCCAGGAGCTAAGT3′/, 5′GCCATAGTAATTCTTGCTCTGC3′ flanking the CASP8 -652 6N ins/del polymorphism, 130 ng of genomic DNA, and 5-µl 2× ImmoMixTM (Bioline, Taunton, MA). PCR cycle reactions were performed on an ABI 2720 Thermal Cycler (Applied Biosystems, Foster City, CA) beginning with a denaturing step at 94°C for 2 min followed by 30 cycles of denaturing at 94°C for 20 sec, annealing at 53°C for 10 sec and extension at 72°C for 15 sec followed by a final extension at 72°C for 5 min.
The primers generated PCR products of either 177 bps (base pairs) (CASP8 -652 6N ins) or 171 bps (CASP8 -652 6N del). The amplified PCR products were digested overnight by the BfaI restriction enzyme (New England Biolabs, Ipswich, MA) The digested PCR products were analyzed on 1.5% agarose gel stained with ethidium bromide using a 25 bp DNA Step Ladder (Promega Corporation, Madison, WI). The samples with CASP8 -652 6N ins variant produced two fragments of 31 bps and 146 bps. The samples with CASP8 -652 6N del allele produced one fragment of 171 bps. Heterozygous samples (CASP8 -652 6N ins/del) produced all the three fragments of 31 bps, 146 bps and 171 bps. The fragment of 31 bps was not detected in the gel analysis due to its small size (Fig. 1).
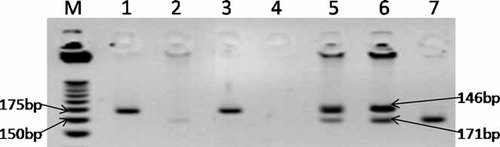
Analysis of the CASP8 -652 6N ins/del polymorphism on agarose gel. M = DNA ladder, samples 1 and 3 = CASP8 -652 6N ins/ins (wild type homozygous), samples 5 and 6 = CASP8 -652 6N ins/del (heterozygous), sample 7 = CASP8 -652 6N del/del (mutant type homozygous), samples 2 and 4 = an example of PCR failure.
DNA sequencing was performed on 40 randomly selected samples to cross-check the genotyping results. The same forward primer (previously used for genotyping of CASP8 -652 6N ins/del) and a new reverse primer (5′CCTCTTCAATGCTTCCTTGAG3′) was used for sequencing of the samples. The DNA sequencing was done using a BigDye Teminator V3.1 Cycle sequencing kit (Applied Biosystems, Foster City, CA) following the manufacturer's protocol. The sequencing results confirmed 100% of the genotyping results.
Statistical Analysis
Multivariate analysis and logistic regression was used to test for correlations and associations of genotype with cervix cancer status as well as other phenotypes such as age, ethnicity and smoking status, and outcomes such as HIV status and abnormal cytology in the control group. Statistical analyses were done using Stata 9 software (College Station, TX).
RESULTS
Genotyping data for CASP8 -652 6N ins/del polymorphism was successful on 99.6% of the cases and 87% of controls of the original starting material. The frequency of CASP8 -652 6N del allele was not significantly different between cases and controls (P = 0.948) but was slightly higher among black Africans (53% in cases and 58% in controls) compared to the Mixed Ancestry (47% in cases and 46% in controls) as shown in Table I. The CASP8 -652 6N ins/del polymorphism was then investigated in the control group with abnormal cytology and high-risk HPV infection. First, a multivariate analysis using multiple regressions were done in order to get evidence of the variables that affected cytology (abnormal versus normal) and HPV types (high risk vs. low risk types). It was observed from multivariate analysis that ethnicity, caspase-8 -652 6N del/del genotype, and HPV significantly influenced cytology (Additional file Ia) while ethnicity and age influenced HPV type (Additional file Ib). In addition, there were ethnic differences in the distribution of the different cytology groupings (i.e., normal, atypical cells of undetermined significance, low-grade squamous epithelial and high-grade squamous epithelial) between Black subjects and Mixed Ancestry subjects (Additional file II). Thus, for subsequent analyses, ethnicity was corrected for. When individuals with abnormal cytology (positive for atypical squamous cells of undetermined significance, LSILs and HSILs) were compared to individuals with normal cytology, a statistically significant association (P = 0.048) was found with CASP8 -652 6N del/del genotype and abnormal cytology (Table II). However, excluding the atypical squamous cells of undetermined significance positive individuals from the abnormal cytology group showed no association (P = 0.182) with CASP8 -652 6N del/del genotype when compared to normal cytology individuals (Table II). Comparing the individuals with high-risk HPV infection with individuals without HPV infection showed a borderline association (P = 0.058) with CASP8 -652 6N del/del genotype and high-risk HPV infection (Table III). When the high-risk HPV infection data was separately analyzed in both the black African and Mixed Ancestry individuals, a statistically significant association (P = 0.030) was found with high-risk HPV infection and CASP8 -652 6N del/del genotype in black Africans (Table III), but not in the Mixed Ancestry group (P = 0.551).
| Genotypes | Controls (n = 1221) | Cases (n = 445) | Genotype-cervical cancer association, adjusted for ethnicity and smoking | |||
|---|---|---|---|---|---|---|
| Black 257 (21), n (%) | Mixed-ancestry 964 (79), n (%) | Black 106 (24), n (%) | Mixed-ancestry 339 (76), n (%) | P-value | OR (95% CI) | |
| CASP8 -652 6N ins→6N del | ||||||
| Ins/ins | 43 (17) | 265 (26) | 18 (17) | 84 (25) | — | 1 |
| Ins/del | 129 (50) | 510 (53) | 63 (59) | 188 (55) | 0.247 | 0.85 (0.65–1.12) |
| Del/del | 85 (33) | 189 (21) | 25 (24) | 67 (20) | 0.948 | 1.01 (0.73–1.41) |
- P-values and OR (95% confidence intervals) are for test of genotype association with cervix cancer risk, adjusted for ethnicity and smoking. P-values next to genotype names are for joint model, others are for ORs of specific genotype compared to reference genotype, indicated with OR = 1. Cases, women with cancer of the cervix (ICC); controls, women without cancer of the cervix; n, counts.
| Genotypes | Normal cytology (n = 969) | Abnormal cytology (n = 161) | Genotype-cervical cancer association, adjusted for ethnicity and smoking | |||
|---|---|---|---|---|---|---|
| Black 177 (18), n (%) | Mixed-ancestry 792 (82), n (%) | Black 56 (35), n (%) | Mixed-ancestry 105 (65), n (%) | P-value | OR (95% CI) | |
| CASP8 -652 6N ins→6N del | ||||||
| Ins/ins | 32 (18) | 227 (29) | 7 (13) | 23 (22) | — | 1 |
| Ins/del | 95 (54) | 408 (51) | 23 (41) | 62 (59) | 0.165 | 1.37 (0.88–2.14) |
| Del/del | 50 (28) | 157 (20) | 26 (46) | 20 (19) | 0.048 | 1.66 (1.00–2.75) |
| CASP8 -652 6N ins→6N del *(minus ASCUS) | ||||||
| Ins/ins | 32 (18) | 227 (29) | 3 (12) | 13 (25) | — | 1 |
| Ins/del | 95 (54) | 408 (51) | 10 (38) | 29 (56) | 0.604 | 1.17 (0.64–2.15) |
| Del/del | 50 (28) | 157 (20) | 13 (50) | 10 (19) | 0.182 | 1.58 (0.81–3.10) |
- Abnormal cytology, positive for pap smear test (i.e., positive for ASCUS + positive for LSIL + positive for HSIL); normal cytology, negative for pap smear test; *minus ASCUS, because ASCUS is not well defined, the respective samples were removed from the total of Abnormal to see whether there was any difference.
| Genotypes | High-risk HPV negative (n = 933) | High-risk HPV positive (n = 197) | Genotype-cervical cancer association, adjusted for ethnicity and smoking | Genotype-cervical cancer association for Black controls, adjusted for smoking | ||||
|---|---|---|---|---|---|---|---|---|
| Black 173 (19), n (%) | Mixed-ancestry 760 (81), n (%) | Black 60 (30), n (%) | Mixed-ancestry 137 (66), n (%) | P-value | OR (95% CI) | P-value | OR (95% CI) | |
| CASP8 -652 6N ins→6N del | ||||||||
| Ins/ins | 32 (19) | 213 (28) | 7 (12) | 37 (27) | — | 1 | — | — |
| Ins/del | 94 (54) | 400 (53) | 24 (40) | 70 (51) | 0.964 | 1.01 (0.68–1.49) | 0.793 | 1.13 (0.44–2.90) |
| Del/del | 47 (27) | 147 (19) | 29 (48) | 30 (22) | 0.058 | 1.53 (0.99–2.38) | 0.030 | 2.84 (1.11–7.28) |
- High-risk HPV negative, negative for hybrid capture II HPV test; high-risk HPV positive, positive for hybrid capture II HPV test.
DISCUSSION
Not much is known about the genetic variations in the CASP8 gene and their role in human cancer susceptibility although the polymorphism has been associated with several pathological conditions including different types of cancers in different populations with conflicting results [Sun et al., 2007; Cybulski et al., 2008; Frank et al., 2008; Li et al., 2008; Pittman et al., 2008; Yang et al., 2008; De et al., 2009; Ji et al., 2009; Ni et al., 2009; Wang et al., 2009]. The CASP8 -652 6N ins/del polymorphism has also been reported to influence the susceptibility to cervical cancer and it is thought that the resultant reduction/decrease in caspase-8 transcription which is associated with increased tumor progression [Sun et al., 2007]. Biochemical analyses have also shown that T lymphocytes with the deletion variant display reduced caspase-8 activity and reduced activation-induced cell death when stimulated with cancer cell antigens [Sun et al., 2007].
This is the first study reporting the frequency of the CASP8 -652 6N ins/del polymorphism in an indigenous black African as well as in a Mixed Ancestry population. No significant association of CASP8 -652 6N del/del genotype and cervical cancer (P = 0.948) was found when compared to control women in black African and Mixed Ancestry population. It has been previously reported that mutated form of caspase-8 acts in a dominant-negative manner preventing the recruitment of the wild type form of caspase-8 to death receptors [Mandruzzato et al., 1997; Kim et al., 2003]. This results in a blockage of signal transduction via the death receptor and apoptotic cell death pathway [Mandruzzato et al., 1997; Kim et al., 2003]. This is supported by the observation of increased likelihood of having abnormal cytology (atypical squamous cells of undetermined significance + LSILs + HSILs) (P = 0.048) among carriers of the CASP8 -652 6N del/del genotype compared to individuals with normal cytology. High-risk HPV infection also showed a borderline significant effect (P = 0.058) with CASP8 -652 6N del/del genotype. However, on separating the two ethnic groups, the black subjects presented with significantly higher risk of HPV infection (P = 0.030) if they were carriers of the CASP8 -652 6N del/del genotype. As the association of this genotype is not observed with the cervical cancer patients, this suggests that majority of the CASP8 -652 6N del/del genotype carriers clear the HPV infection and the pre-cancerous lesions regress. This would imply that those with the CASP8 -652 6N del/del genotype do not progress to cancer of the cervix with a mechanism hitherto unknown. The shift in roles of CASP8 -652 6N del/del genotype possibly happens somewhere during the early stages of pre-cancer development including HPV infection.
Caspase-8 long (caspase-8L) that results from a 136-bp deletion is the most common of all the existing caspase-8 isoforms [Mohr et al., 2005]. It lacks proteolytic activity and does not facilitate signal transduction from activated death receptors acting in a dominant-negative manner [Fulda, 2009]. Thus, caspase-8L distribution in different populations may affect the observed effects of the CASP8 -652 6N ins/del polymorphism. Its distribution in the current study population is not known. More studies are needed to tease out what could be the contributory factor in the observed ethnic differences as well as non-association of the CASP8 -652 6N with cervical cancer, an observation reported in this study. It is important to note that CASP8 -652 6N deletions, is not the only way caspase-8 expression is lost. A recent report shows that DNA methylation of CASP8 gene is also used to switch off caspase-8 expression [Wu et al., 2010]. Thus, this study might not have found a difference in the distribution of the CASP8 -652 6N ins/del between cases and controls but the actual expression levels might be significantly different. Though there is evidence that genetic frequency difference in different races do not generally influence their biological impact on a disease [Ioannidis et al., 2004], the possibility still remains. The presence of another causative mutation in tight LD with this polymorphism can also not be ruled out.
However, it has been shown that the death receptor pathway behaves aberrant in early stages of pre-carcinogenic lesions, dysplasia, and carcinoma in situ [Gratas et al., 1998; Kase et al., 2002]. It is possible that during early stages of cancer development the caspase-cascade, mediated mainly by the death receptor pathway activates apoptosis in malignant cells. This will eliminate the malignant cells and slow down the process of cancer development. Individuals carrying the CASP8 -652 6N del/del variant will express less caspase-8 hence less apoptosis of the malignant cells leading to a faster progression of tumor compared to the individuals carrying the CASP8 -652 6N ins/ins variant of it. The results showing a significant susceptible effect with abnormal cytology (Table II) and with high-risk HPV infection (Table III) with the deletion variant of CASP8 (CASP8 -652 6N del/del) support this hypothesis.
In conclusion, this study did not show any significant association of CASP8 -652 6N ins/del polymorphism with cervical cancer, but showed a statistically significant association with abnormal cytology in black African and Mixed Ancestry individuals. This study also showed a significant effect of this polymorphism with high-risk HPV infection in black Africans. CASP8 -652 6N del/del genotype increased the risk of developing abnormal cytology in black African and Mixed Ancestry individuals and increased the risk of high-risk HPV infection in black African individuals. Further studies are needed with patients in different stages of cervical cancer to confirm these findings.
Acknowledgements
We are grateful to all the patients and controls who participated in this study. This work is based upon research supported by the South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation. It was also supported by the NRF South Africa/Sweden Science and Technology Agreement's Fund. Experimental Ethics: All experiments were performed in compliance with relevant laws and institutional guidelines (University of Cape Town Human Research Ethics Committee study approval number, REC REF: 075/2009) and in accordance with the ethical standards of the Declaration of Helsinki. Informed Consent: Written informed consent was obtained from each participant of this study.




