Etiological role of human papillomavirus infection for inverted papilloma of the bladder†
The institution at which the work was performe; Kanazawa University Graduate School of Medical Science, Kanazawa, Japan.
Abstract
The status of human papillomavirus (HPV) infection in urothelial inverted papilloma was examined in the present study. Formalin-fixed and paraffin-embedded tissues from eight cases of inverted papilloma of the bladder were studied. The presence of HPV-DNA was examined by modified GP5/6+PCR using archival tissue sections by microdissection. HPV genotype was determined with a Hybri-Max HPV genotyping kit. Immunohistochemical analysis for p16-INK4a, mcm7, HPV-E4, and L1, and in situ hybridization for the HPV genome were performed. HPV was detected in seven of eight cases (87.5%) of inverted papilloma. Three cases were diagnosed as inverted papilloma with atypia, while the remaining five were typical cases. HPV-18 was detected in two cases, including one inverted papilloma with atypia, and HPV-16 was detected in four cases, including one inverted papilloma with atypia. Multiple HPV type infection was detected in one typical case and one atypical case. High-risk HPV was present in all HPV-positive cases. Cellular proteins, p16-INK4a and mcm7, which are surrogate markers for HPV-E7 expression, were detected in all HPV-positive cases, and their levels were higher in inverted papilloma with atypia than in typical cases. In contrast, HPV-E4 and L1, which are markers for HPV propagation, were observed in some parts of the typical inverted papilloma tissue. High-risk HPV infection may be one of the causes of urothelial inverted papilloma, and inverted papilloma with atypia may have malignant potential. J. Med. Virol. 83:277–285, 2011. © 2010 Wiley-Liss, Inc.
INTRODUCTION
Certain types of human papillomavirus (HPV), which are transmitted through sexual contact, are causative agents of cervical cancer and possibly some other cancers. HPV is highly prevalent in sexually active young men and women. However, many studies have suggested that men only act as a reservoir for HPV transmission, as urothelial malignancies are considered to be induced rarely by HPV. However, HPV has been detected in some cases of urothelial carcinoma in the bladder [Barghi et al., 2005; Moonen et al., 2007]. Bladder cancer generally develops in men over 60 years old in contrast to cervical cancer in younger women, suggesting that HPV is unlikely to induce bladder cancer with a high incidence rate. Indeed, HPV-DNA detection is much less frequent in bladder cancer than in cervical cancer, and various rates of positivity for HPV-DNA in bladder cancer have been reported [Kamel et al., 1995; Tenti et al., 1996; Barghi et al., 2005; Moonen et al., 2007]. Thus, the etiological role of HPV in bladder cancer is still controversial.
Inverted papilloma is a rare benign urothelial tumor that develops predominantly in the bladder, and accounts for <1% of all urothelial neoplasms [Sung et al., 2006b]. This type of tumor is characterized pathologically by inverted growth of transitional epithelia with no atypia. Generally, inverted papilloma occurs more frequently in men than in women, and the most common target age is 30–60 years old, which is younger than bladder cancer [Sung et al., 2006b]. Although the etiological factors associated with inverted papilloma have not been established, HPV-DNA has been detected in some cases of inverted papilloma [Chan et al., 1997]. A recent study reported a high prevalence (35%) of HPV infection in the urinary tracts (urethra and urine) of 142 men with urethritis [Shigehara et al., 2010], and morphological abnormal cells suggestive of high-risk HPV infection were identified on Papanicolaou test of scraped cells from the lower segment of the urethra, suggesting that persistent HPV infection in the urinary tract may be a cause of bladder neoplasm.
In the present study, the presence of HPV-DNA in the tissue of inverted papilloma of the bladder was examined using a microdissection procedure, and the status of HPV infection in the tissue was also investigated using an immunohistochemical (IHC) method with detection of HPV-related proteins and by in situ hybridization (ISH) analysis for HPV-DNA.
MATERIALS AND METHODS
Patients
Eight cases of inverted papilloma of the bladder treated at Kanazawa University Hospital between 2000 and 2009 were investigated in this study. Histopathological diagnosis of inverted papilloma was made based on 2004 World Health Organization classification of noninvasive urothelial tumors [Lopez-Beltran and Montironi, 2004]. Written informed consent for usage of these samples had already been obtained from all patients before surgical resection, in accordance with the protocol approved by the Ethics Committee of Kanazawa University Graduate School of Medicine.
Samples
The inverted papilloma tissues were fixed immediately in 20% formalin, embedded in paraffin, and stored at room temperature. The cases were classified pathologically into two groups: The first was composed of normal urothelial cells (classical or typical inverted papilloma), showing thin anastomosing trabeculae of urothelium that grow downward into the stroma and absence of a papillary component; and the second was inverted papilloma with atypia (atypical inverted papilloma), which had foci of atypia in a background of classic inverted papilloma, as reported previously [Broussard et al., 2004]. Seven paraffin-embedded normal mucosal samples, which were obtained by random bladder biopsy from another seven patients with inflammatory bladder disorder, were used as negative controls.
HPV-DNA Analysis and Typing
DNA was extracted from paraffin-embedded inverted papilloma tissue and controls by microdissection using the Pinpoint Slide DNA Isolation System™ (Zymo Research, Orange, CA). The extracted DNA was stored at −30°C until use.
DNA quality was confirmed by amplifying the β-globin gene as an internal control by PCR. HPV-DNA was amplified using a modified GP5+/GP6+PCR method as previously reported [Yamada et al., 2008]. HPV was defined as positive when a band of about 140 bp was observed after electrophoresis of 8 µl of PCR product on 2.0% agarose gels and staining with ethidium bromide.
HPV genotyping was performed using an HPV GenoArray Test Kit (HybriBio Ltd., Chaozhou, China). This assay can determine 21 HPV types, including 14 high-risk HPV types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68), five low-risk HPV types (6, 11, 42, 43, and 44), and two unknown-risk types (53 and CP8304), by the flow-through hybridization technique using HPV-DNA amplified by PCR [Shigehara et al., 2010].
In Situ Hybridization for HPV-DNA
ISH was performed to detect HPV-DNA in tumor tissue using an HPV-detection kit according to the manufacturer's instructions (Dako GenoPoint System K0620; Dako, Carpinteria, CA). A wide-spectrum probe (Y1404; Dako) for 13 high-risk HPV-DNA (HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68), and low-risk HPV-DNA (HPV types 6, 11) probe (Y1411; Dako), and HPV types 16, 18 probe (Y1412; Dako) were hybridized with denatured DNA on tissue samples. HPV-DNA signals were visualized as brownish staining. Hematoxylin was used to counterstain the cell nuclei in all specimens.
Immunohistochemistry for HPV-Related Proteins
The procedure for IHC staining was performed using a Dako ChemMate ENVISION Kit/HRP(DAB)-universal kit (K5007) according to the manufacturer's protocol (Dako). Tissue specimens were stained with mouse monoclonal antibodies against p16-INK4a (Immuno-Biological Laboratories Co., Gunma, Japan) and against mini chromosome maintenance protein-7 (mcm-7) (Abnova, Taipei, Taiwan), and against HPV-L1 protein (Viroactiv; Virofem Diagnostica, Mainz, Germany). HPV-E4 staining was performed using a type-specific rabbit polyclonal antibody against E4 proteins of HPV-6, -11, -16, -18, and -33 according to the protocol previously described [Doorbar, 2006].
Antigen retrieval was performed by heating tissue sections at 95°C in Retrieval Solution, pH 9 (S2367; Dako) for 40 min. After blocking endogenous peroxidase activity using 3% hydrogen peroxide for 5 min, the sections were incubated with the primary antibodies at room temperature for 30 min, followed by incubation for 30 min with peroxidase-labeled secondary antibody (a mixture of rabbit and mouse antibodies) combined with dextran-polymers. Brownish staining for target proteins on tissue slides was developed using DAB, and counterstained with hematoxylin. The expression of each protein was scored according to the following scale: Distribution of signals was defined as 0 (<5% of the cells were positive), 1+ (some: 5–30% of the cells were positive), 2+ (many: 30–50% of the cells were positive), and 3+ (extensive: ≧50% of the cells were stained). The intensity of the signals was as 0 (not stained), 1+ (moderately stained), and 2+ (strongly stained). We calculated the score as multiplying the distribution score and the intensity score, and the expression levels of p16 and mcm7 in typical and atypical inverted papilloma were compared.
RESULTS
Detection of HPV Genomes in Inverted Papilloma Tissue
Eight cases of inverted papilloma were investigated, and all results are summarized in Table I. Five cases (cases 3, 4, 5, 7, and 8) were diagnosed as typical inverted papilloma characterized by anastomosing cords and thin nests of normal urothelium growing down from the surface of the epithelium, and often formed glandular or gland-like structures (Fig. 1A,B). In contrast, three cases (cases 1, 2, and 6) were composed of various degrees of abnormal cell nests containing cells of increased chromatin, irregular nuclear contours, and increased nuclear/cytoplasmic ratios, with a loss of cell polarity (Fig. 1C,D).
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | |
|---|---|---|---|---|---|---|---|---|
| Age | 36 | 58 | 35 | 49 | 78 | 37 | 66 | 53 |
| Sex | Male | Male | Female | Male | Male | Male | Male | Male |
| Pathological finding | Atypical | Atypical | Typical | Typical | Typical | Atypical | Typical | Typical |
| Bglobin | + | + | + | + | + | + | + | + |
| HPV type | 18 | 33,58,68 | 6,16,33 | 16 | 18 | 16 | 16 | Negative |
| In situ hybridization | ||||||||
| High-risk HPV probe | 2+ (punctate) | 2+ (punctate) | 1+ (diffuse) | 1+ (diffuse) | 1+ (diffuse/punctate) | 1+ (punctate) | 2+ (diffuse) | − |
| Type 6/11 probe | − | − | 2+ (diffuse) | − | − | − | − | − |
| Type 16/18 probe | 2+ (punctate) | − | +/− (diffuse) | 2+ (diffuse) | 2+ (diffuse/punctate) | 2+ (punctate) | 2+ (diffuse) | − |
| Immunohistochemistory (area/intensity/location) | ||||||||
| p16 | 3+/3+/N | 3+/2+/N | 2+/2+/N | 2+/3+/N | 2+/2+/N | 3+/2+/N | 2+/1+/NC | −/−/− |
| mcm7 | 2+/3+/NC | 2+/2+/NC | 1+/2+/NC | 2+/2+/NC | 1+/1+/NC | 2+/3+/NC | 1+/2+/NC | −/−/− |
| HPV-E4 type11 (control) | −/−/− | −/−/− | −/−/− | −/−/− | −/−/− | −/−/− | −/−/− | −/−/− |
| Type6 | a | a | 3+/2+/C | a | a | a | a | a |
| Type16 | a | a | 2+/2+/NC | 2+/1+/C | a | 3+/2+/N | 1+/1+/NC | a |
| Type18 | +/+/NC | a | a | a | 3+/1+/C | a | a | a |
| Type33 | a | 2+/2+/C | 2+/1+/N | a | a | a | a | a |
| HPV-L1 | +/+/C | +/+/C | +/+/C | 2+/1+/C | −/−/− | +/+/C | −/−/− | −/−/− |
- Immunohistochemical; signal area: −, <5%; 1+, <5–30%; 2+, 30–50%; 3+, ≧50%; signal intensity: −, negative; 1+, moderate; 2+, strong signal location; N, nucleus; C, cytoplasm; NC, nucleus and cytoplasm.
- a Not examined.
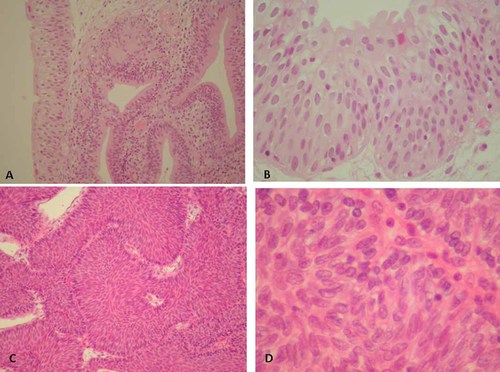
Histological findings of inverted papilloma stained with hematoxylin & eosin (HE). A: Gland-like structure in typical inverted papilloma of case 3 (HE staining, magnification ×100). B: Typical inverted papilloma of case 3 (HE staining, magnification ×400). C: Inverted papilloma with atypia of case 1 (HE staining, magnification ×100). D: Inverted papilloma with atypia of case 2 (HE staining, magnification ×400). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com]
HPV-DNA was detected in seven cases (87.5%). HPV-16 was identified in four cases, including three typical and one atypical inverted papilloma. HPV-18 was detected in one typical and one atypical inverted papilloma. Multiple HPV type infection was observed in two cases (one typical and one atypical inverted papilloma). The former was positive for HPV types 6, 16, and 33, and the latter was positive for HPV types 33, 58, and 68. High-risk HPV types were identified in all of HPV-positive cases.
ISH analysis revealed the presence of high-risk HPV-DNA in the nuclei of most of the tumor cells in all HPV-positive cases (Fig. 2A). Different signal patterns were observed between typical and atypical inverted papilloma. Punctate signals, which may represent HPV-DNA integration, were predominant in atypical inverted papillomas (Fig. 2B, Table I), whereas diffuse and intense signals, which are due to plasmid state of HPV-DNA, were seen in typical inverted papilloma (Fig. 2C, Table I). HPV-DNA signals for HPV-16 and -18 were detected in cases 1, 3, 4, 5, 6 and 7. On the other hand, no HPV-DNA signals were detected in one HPV-negative case. HPV-6 and -11 signals were seen only in case 3 infected with HPV-6 (Fig. 2D, Table I). The HPV type determined with ISH was consistent with that indicated by the HPV genotyping test. Both PCR and ISH analysis demonstrated that HPV-DNA was not detected in any control bladder specimens.
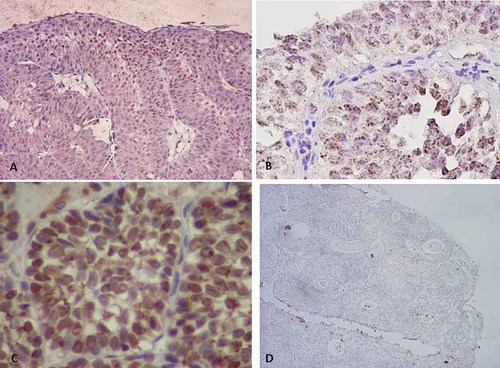
Localization and signal patterns of HPV-DNA in the inverted papilloma (inverted papilloma) by in situ hybridization analysis. A: High-risk HPV-DNA signals in typical inverted papilloma of case 3 (magnification ×100). B: Punctate signals of high-risk HPV-DNA in inverted papilloma with atypia of case 2 (magnification ×400). C: Diffuse and intense signals of HPV-16/18-DNA in typical inverted papilloma of case 6 (magnification ×400). D: No signals of HPV type 6/11-DNA in inverted papilloma of case 5 (magnification ×40). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com]
Expression of Cellular Proteins p16 and mcm7, which are Surrogate Markers of High-Risk HPV-E7 Protein, in Inverted Papilloma
Cellular protein p16 is an inhibitor of cyclin-dependent kinase that stops the cell cycle. The expression can be enhanced by high-risk HPV-E7 protein, and the cell cycle inhibitory function of p16 is over-ridden in E7-expressing cells [Klaes et al., 2001; Shai et al., 2007]. Expression of mcm7 is also induced by E7 and contributes to cellular DNA replication [Brake et al., 2003]. These cellular proteins are therefore regarded as surrogate markers for the expression of E7 protein of high-risk HPV type in uterine cervical intraepithelial neoplasia (CIN) and cervical cancer. Both p16 and mcm7 were expressed in all HPV-positive cells in both typical and atypical inverted papilloma (Fig. 3A,B). The p16 and mcm7 signals were observed predominantly in the nucleus, and mcm7 was expressed faintly in the cytoplasm in some cells. The p16-positive inverted papilloma cells were observed more frequently than those positive for mcm7. Neither p16 nor mcm7 signal was observed in one case, which was negative for HPV-DNA, or in seven control specimens (Fig. 3C,D).
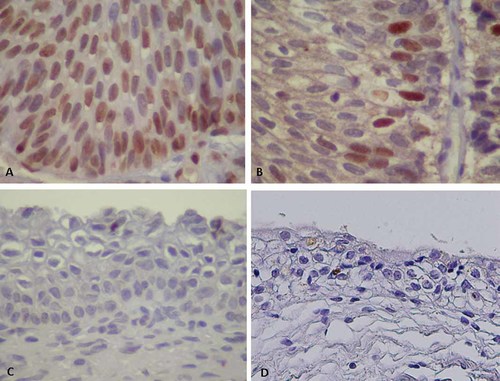
Expression patterns of p16 and mcm7 proteins in the inverted papilloma (inverted papilloma) (magnification ×400). A: The p16 signals in the nuclei of inverted papilloma cells of case 1. B: The mcm7 signals in the nucleus of inverted papilloma cells of case 1. C: No signals of mcm7 in control transitional cells. D: No signals of p16 in control transitional cells. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com]
The mean expression levels of these proteins were compared using a scoring system that recorded the distribution of the signals. Significant higher scores for mcm7 and marginally higher scores for p16 were observed in atypical than in typical inverted papilloma (Fig. 4). The levels of p16 or mcm7 expression represent proliferative activity induced by HPV infection, suggesting that atypical inverted papilloma may be more aggressive than typical cases.
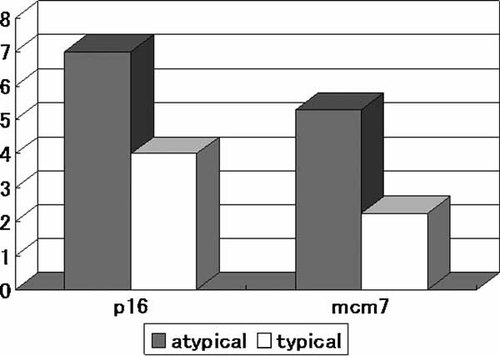
Comparison of expression levels of p16 and mcm7 proteins in inverted papilloma with atypia and typical inverted papilloma (in seven HPV-positive cases) (Mann–Whitney U-test; P < 0.05).
HPV Infection Status in Inverted Papilloma
There are two types of HPV infection, for example reproductive and abortive infection. The HPV-E4 protein is able to recognize the keratin network in host cells and stop cell division, which may facilitate the release HPV particles [Doorbar, 2006]. This protein is expressed abundantly during reproductive infection. HPV-E4 protein for each HPV type, such as HPV-6, -16, -18, and -33, was detected in all HPV-positive inverted papillomas (Table I, Fig. 5A–C), while no signals were seen for HPV-11-E4 protein (Table I, Fig. 5D). The E4 antibody was HPV type specific. HPV-E4 was expressed more strongly in the cytoplasm of typical inverted papilloma (Fig. 5C) than in atypical inverted papilloma (Fig. 5A). Curiously, nuclear staining was observed occasionally in some cases (Fig. 5B). HPV-E4 protein was not expressed in one HPV-negative inverted papilloma or in any controls.
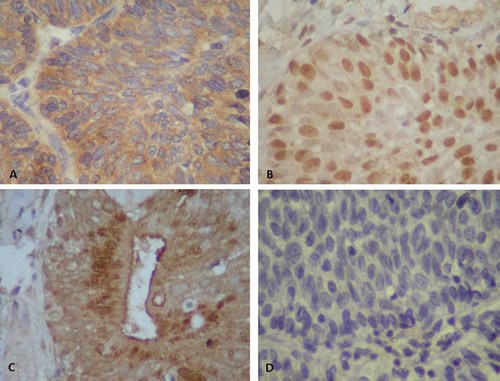
Expression patterns of HPV-E4 protein in inverted papilloma (inverted papilloma) (magnification ×400). A: Abundant expression of HPV-18-E4 in the cytoplasm of inverted papilloma with atypia of case 1. B: HPV-16-E4 expression in the nuclei of inverted papilloma of case 3. C: Expression of HPV-6-E4 protein in the cytoplasm of apical parts of gland-like structures in inverted papilloma of case 3. D: No signals for HPV-11-E4 in inverted papilloma of case 1 (negative control). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com]
HPV-L1 protein is the major capsid protein that assembles to form the viral shell. L1 protein was expressed widely only in case 4 (Fig. 6A), while a low level of expression was seen in limited parts of the inverted papilloma tissue of cases 1, 2, 3, and 6. The expression was limited to the apical sites of glandular structures and superficial layers of transitional cell epithelium in inverted papilloma (Fig. 6B,C). L1 protein expression was not observed in one HPV-18-positive case (case 5) and one HPV-16-positive case (case 7), although these were typical inverted papilloma cases. Furthermore, this protein was not detected in atypical sites of atypical inverted papilloma, one HPV-negative inverted papilloma, or control urothelial cells (Fig. 6D).
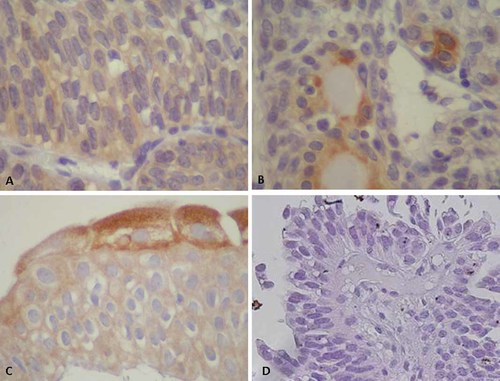
Expression patterns of high-risk HPV-L1 protein in the inverted papilloma (inverted papilloma) (magnification ×400). A: Abundant cytoplasmic expression of L1 in inverted papilloma of case 1. B: L1 expression in the apical parts of gland-like structures of inverted papilloma of cases 2. C: L1 expression in the superficial layers of transitional cell epithelium within inverted papilloma of case 4. D: This protein was not expressed in atypical parts of inverted papilloma with atypia (case 1). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com]
DISCUSSION
Potts and Hirst [1963] first reported a benign bladder tumor designated as inverted papilloma. Traditionally, inverted papilloma has been regarded as a benign neoplasm or a hyperplastic reactive lesion. However, cases with recurrence or those with synchronous or metachronous urothelial carcinoma have generated uncertainly with regard to its malignant potential. A systematic review of 322 cases of inverted papilloma in the bladder indicated that the recurrence rates was 4% and the incidences of associated synchronous urothelial carcinoma and development of subsequent urothelial carcinoma were 6% and 3%, respectively [Cheng et al., 2005]. Overaccumulation of p53, overexpression of HER-2/neu, and increased proliferation index have been identified in some inverted papillomas, suggesting that it may be susceptible to malignant transformation [Cheon et al., 1995; Urakami et al., 1996]. However, the association of inverted papilloma with such molecular events is less frequent than in bladder urothelial carcinoma [Sung et al., 2006a]. Some studies have doubted the malignant potential of inverted papilloma, as its recurrence is very rare and inverted papilloma with atypia does not show high proliferation activity [Broussard et al., 2004; Sung et al., 2006a].
The etiology of inverted papilloma has not been determined. One study suggested an association between HPV infection and inverted papilloma of the bladder [Chan et al., 1997]. In this previous study, in which the presence of the HPV genome was examined by HPV type-specific PCR for HPV types 6, 11, 16, 18, 31, and 33, HPV was detected in 60% (6/10) of cases of inverted papilloma. HPV-16 and -18 were detected in one and six cases, respectively. The results of the present study indicated a strong association between high-risk HPV infection and inverted papilloma, as high-risk HPV-DNA was identified in seven (87.5%) of eight cases of inverted papilloma. Furthermore, the localization of HPV-DNA was confirmed by ISH analysis using specific probes for HPV-16, -18, and high-risk HPV types. HPV-16 was the most common type. Low-risk HPV that is HPV-6 was found in one case of inverted papilloma, but this case showed co-infection with two high-risk HPV types. These findings suggested that high-risk HPV is likely to be one of the causes for the development of inverted papilloma. A recent study indicated a high prevalence rate (35%) of high-risk HPV infection in the lower urinary tract (urethra and urine) in 142 male patients with urethritis, suggesting that incidental HPV infection occurs not only in the external genitalia but also in the lower urinary tract [Shigehara et al., 2010]. It is not surprising that HPV could infect urothelium in the urinary bladder and consistent HPV infection may result in the development of urothelial neoplasm.
Pathologically, two different entities were observed among the present cases of inverted papilloma. Some cases had foci of atypical urothelium composed of atypical cells with nuclear enlargement, increased chromatin, and thickening of the nuclear membrane, in a background of the classic inverted papilloma. Such cases were reported as inverted papilloma with atypia [Broussard et al., 2004]. On the other hand, typical inverted papilloma was composed of anastomosing cords and thin nests of normal urothelium growing down from the surface epithelium, and sometimes accompanied with gland-like or glandular structures. In the present ISH analysis of HPV-DNA, diffuse (cases 3, 4, and 7), punctate (cases 1, 2 and 6), and mixed diffuse and punctate staining patterns (case 5) were observed in the nuclei of inverted papilloma cells. It has been reported that the diffuse patterns represent the episomal state of the HPV genome in host cells, while punctate patterns indicate integration of the HPV genome into the host cells [De Marchi Triglia et al., 2009]. Integration is observed frequently in high-grade CIN and cervical cancer [Kalof et al., 2005; De Marchi Triglia et al., 2009], while the episomal state is seen in papilloma or in lower grade CIN [Cooper et al., 1991]. Interestingly, punctate signals (integration) were observed predominantly in atypical inverted papilloma, while diffuse signals (episomal) were seen in typical cases.
The loss of p16 expression is often a critical event in the progression of tumors in many organs, including bladder cancer [Rocco and Sidransky, 2001]. In fact, some studies have indicated that p16 protein is down-regulated in 19–60% of bladder cancers [Niehans et al., 1999; Yang et al., 2002; Krüger et al., 2005]. In contrast, it is well known that up-regulation of p16 in the presence of HPV-E7 protein plays an important role in the process of carcinogenesis in cervical cancer [Klaes et al., 2001; Shai et al., 2007] and head and neck cancer [Hafkamp et al., 2003]. The mcm7 gene is also responsive to E2F, and is used as another surrogate marker expressed in high-grade CIN and cervical cancer [Brake et al., 2003]. Therefore, we also investigated the expression of p16 and mcm7 proteins, which are surrogate markers of E7 protein. Higher levels expression of p16 and mcm7 indicate higher activity of cell division with high-level E7 protein expression. In the present study, p16 and mcm7 were expressed in all HPV-positive cells in both typical and atypical inverted papilloma, supporting the suggestion that HPV infection may be associated with the development of inverted papilloma of the bladder. Furthermore, p16 and mcm7 were expressed at higher levels in atypical than in typical inverted papilloma. These observations suggest that cell growth is more aggressive for atypical inverted papilloma. The findings of HPV-DNA integration and high-level expression of p16 and mcm7 proteins in atypical inverted papilloma suggested its potential for malignant conversion. The present findings suggest that high-risk HPV may be a causative agent of urothelial inverted papilloma, and atypical inverted papilloma may be a precursor of urothelial cancer. On the other hand, Broussard et al. [2004] excluded the potential of atypical inverted papilloma for malignant progression in experiments regarding Ki-67 and cytokeratin-20 expression in atypical inverted papilloma tissue. It is not surprising that expression levels of these cell proliferation markers are reduced in atypical inverted papilloma in comparison with carcinoma, as atypical inverted papilloma is thought only to be a precursor lesion for cancer.
The main limitation of this study was the small number of specimens. Therefore, it will be important to confirm our hypothesis by further studies with larger numbers of specimens. Further studies are also needed to determine whether expression of p16 and mcm7 coincides with HPV infection in randomized samples of bladder cancer to evaluate the possible etiological role of HPV in urothelial carcinoma.
If HPV is one of the causative factors of inverted papilloma, this would raise the question of whether inverted papilloma is a source of HPV propagation or of abortive infection. In women, HPV infection is thought to be reproductive in condyloma acuminate and CIN 1. However, it changes to an abortive infection in high-grade CIN and cervical cancer after many years of persistent infection. We also investigated the expression of HPV-E4 and L1 proteins that are concordant with cell cycle arrest and HPV propagation [Doorbar, 2006]. E4 protein expression was observed in all cases of HPV-positive inverted papilloma. Although E4 expression is often observed in the cytoplasm of differentiating epithelial cells (Fig. 5A,C), in some situations the expression was apparent in the nucleus of atypical inverted papilloma (Fig. 5B). This may represent a different form of the protein, and it is interesting that N-terminal cleavage of HPV-16 E4, which removes its keratin binding motif, can lead to nuclear accumulation of E4 in experimental systems [Roberts et al., 1997; Wang et al., 2004]. We do not know the exact reason or mechanism for such aberrant expression patterns for E4 in atypical inverted papilloma. On the other hand, L1 protein expression was observed in five cases of inverted papilloma, although it was restricted to typical sites of glandular structures and superficial layers of transitional cells. The signals were not detected in two HPV-positive cases that were typical inverted papilloma. These findings suggested that HPV propagation may occur in limited parts of the inverted papilloma tissue, but its extent may be limited.
In conclusion, high-risk HPV may be one of the etiological factors for inverted papilloma and inverted papilloma with atypia may have malignant potential.




