Presence of valine at position 27 of the hepatitis B virus core gene is associated with severe liver inflammation in Chinese patients
Abstract
Although it is widely believed that cytotoxic T lymphocytes (CTL) are responsible for severe flares of chronic hepatitis B that lead to liver failure, the published evidence to support this hypothesis is weak. The frequency of the I27V mutation in the HBV core gene, which produces a core 18–27 peptide capable of binding HLA-A*02, was compared in Chinese patients with severe liver inflammation (n = 77, including 39 with acute-on-chronic liver failure), moderate liver inflammation (n = 44) and inactive disease (n = 45). The frequency with which V27 reverted to the wild-type I27 was compared in severe liver inflammation patients who were either HLA-A*02 positive (n = 5) or negative (n = 5). The frequency of patients with a V27 positive HBV was higher in severe than in moderate liver inflammation (23.4% vs. 6.8%, P = 0.02) or inactive disease (23.4% vs. 4.7%, P = 0.006). After a minimum of 3 months follow-up, the frequency of reversion of V27 to the wild-type I27 was higher in HLA-A*02 positive than negative patients (5/5 vs. 1/5, P = 0.05). In summary, this is the first data showing an association between a specific amino acid mutation (I27V) and severe liver inflammation in patients with chronic hepatitis B. This mutation would produce a peptide that is known to bind HLA-A*02 and stimulate CTL. The high frequency of reversion to wild-type I27 in HLA-A*02 positive subjects suggests that CTL recognizing this peptide exist, and is consistent with the possibility that they contribute to the pathophysiology of severe liver inflammation in chronic hepatitis B. J. Med. Virol. 83:218–224, 2011. © 2010 Wiley-Liss, Inc.
INTRODUCTION
The most common hepatitis B virus (HBV) genotypes in China are genotypes B and C [Zeng et al., 2005]. These genotypes have an isoleucine (I) at amino acid 27 of the core gene, in contrast to European genotypes A and D [Allain, 2006], which have a valine (V) at position 27 [Bertoletti et al., 1993]. However community-based studies in China have shown that V27 does occur in HBV of both genotypes B and C, with frequencies of 1.9% [Liu et al., 2007] and 6.7% [Ma et al., 2008] having been reported.
A recent survey of HBV core gene sequences in patients who had been referred to Nanfang Hospital, China for assessment of their liver disease revealed a surprisingly high frequency (13.9%) of subjects with the V27 mutation relative to the previous community-based studies (data not shown). This observation provided an opportunity to test two hypotheses. The first hypothesis is that V27 contributes to the pathogenesis of the severe liver inflammation that is found in this in-patient cohort. The second hypothesis is that any effect on liver inflammation that might be identified is due to CD8+ T cells responding to the core 18–27 peptide (FLPSDFFPSV) presented by an HLA-A*02 allele [Bertoletti et al., 1994]. The core 18–27 peptide with a valine at position 27 (V27) has a higher affinity for HLA-A*02 than the peptide with an isoleucine (I27) at position 27 [Bertoletti et al., 1994]. This raises the possibility that an I27V mutation that arose in a Chinese patient as a result of genetic drift could produce a cytotoxic T lymphocyte (CTL) response to the new epitope that precipitated severe liver inflammation.
The first hypothesis was tested by comparing the frequency of the V27 mutation in HBV extracted from the serum of patients with inactive disease, moderate liver inflammation, or severe liver inflammation. The second hypothesis was tested by looking for evidence of positive selection pressure on V27 in patients who were HLA-A*02 positive.
PATIENTS AND METHODS
Patients
Thirty-nine patients with acute-on-chronic liver failure (ACLF) due to hepatitis B and 38 patients with severe hepatitis B (SHB) were admitted to Nanfang Hospital between January 2008 and July 2009. These two groups comprised the severe liver inflammation group of the study. The SHB group had a serum alanine aminotransferase (ALT) of ≥600 IU/L, a total bilirubin (TB) of ≥3.0 mg/dl, and plasma prothrombin activity (PTA) of ≤50% [Imamura et al., 2003]. The diagnosis of ACLF was based on clinical evidence of either ≥grade 2 hepatic encephalopathy (n = 15), abrupt and obvious increase in ascites (n = 12), spontaneous bacterial peritonitis (n = 4) or hepatorenal syndrome (n = 8). These clinical criteria were associated with either the recent development of jaundice (TB ≥ 10.0 mg/dl) or rapidly rising levels of TB (TB ≥ 1.0 mg/dl/day), and a PTA ≤ 40% [Sarin et al., 2009]. Residual serum from a routine blood sample taken at presentation was used for analysis of the HBV core gene in all 77 subjects with severe liver inflammation. Heparinized blood for determination of HLA-A*02 status was collected from 64 of the 77 patients with severe liver inflammation within a week of presentation. This sample was not taken from very ill patients. In addition, serum and PBMC were available on at least one occasion from 30 of these 64 subjects at more than 3 months after discharge. All patients were discharged on anti-viral therapy, with normal ALT levels and low levels of HBV DNA. Subject 27 was non-compliant after discharge, and had a high ALT level at a 12-month follow-up visit.
The moderate liver inflammation group was comprised of 44 out-patients with CHB who presented to Nanfang Hospital between 2008 and 2009. The CHB patients had elevated ALT levels (80–400 IU/L) and a TB ≤ 1.0 mg/dl. Serum for HBV DNA analysis was stored at presentation. The inactive disease group was comprised of 20 subjects with an inactive, HBeAg-positive chronic HBV infection (high HBV DNA and normal ALT levels), and 60 subjects with an inactive, HBeAg-negative chronic HBV infection (HBV DNA < 1,000 copies/ml and normal ALT levels). They were recruited from a cohort of inactive disease patients who have routine screening for active hepatitis and hepatocellular carcinoma. HBV DNA could not be extracted from 35 of the 60 HBeAg-negative subjects.
All subjects had been HBsAg-positive for at least 1 year. Co-infection by the human immunodeficiency virus (HIV), hepatitis A virus (HAV), hepatitis C virus (HCV), hepatitis D virus (HDV), and hepatitis E virus (HEV) was excluded by laboratory testing. There was no clinical or imaging evidence of liver cirrhosis in any subject. The HBeAg status, age, gender, ALT and HBV DNA levels, HBV genotypes and the presence/absence of precore and core promoter mutations are summarized in Table I. Written informed consent for participation in the study was obtained from all subjects, or from first-degree relatives of patients with severe encephalopathy.
| CHB | SHB | ACLF | Inactive disease | |||||
|---|---|---|---|---|---|---|---|---|
| HBeAg+ | HBeAg− | HBeAg+ | HBeAg− | HBeAg+ | HBeAg− | HBeAg+ | HBeAg− | |
| No. | 36 | 8 | 21 | 17 | 11 | 28 | 20 | 25 |
| Age (years) | 26 ± 6 | 28 ± 5 | 35 ± 8 | 34 ± 10 | 32 ± 7 | 38 ± 10 | 25 ± 7 | 32 ± 9 |
| Gender (M/F) | 29/7 | 7/1 | 17/4 | 16/1 | 9/2 | 27/1 | 14/6 | 16/9 |
| HBV DNA (log10 copies/ml) | 9.4 ± 1.6 | 8.0 ± 2.0 | 5.9 ± 1.7 | 5.1 ± 1.5 | 5.8 ± 1.7 | 5.7 ± 1.8 | 6.8 ± 1.1 | 3.2 ± 0.7 |
| Precore mutationa | 24/9 | 1/3 | 9/6 | 10/4 | 2/5 | 11/12 | 18/2 | 6/14 |
| G1896A (Neg/Pos) | 32/4 | 5/3 | 16/5 | 5/12 | 8/3 | 13/15 | 19/1 | 12/13 |
| Genotype (B/C/D)b | 22/14/0 | 0/8/0 | 12/9/0 | 14/1/0 | 6/2/1 | 18/8/0 | 14/6/0 | 19/5/0 |
| ALT (IU/L) | 125 ± 83 | 143 ± 62 | 519 ± 685 | 726 ± 711 | 482 ± 387 | 623 ± 560 | 32 ± 16 | 21 ± 6 |
- Numerical data are shown as the mean ± SD.
- a The precore promoter could not be amplified in 30 subjects.
- b Amplification of the S ORF for genotyping failed in seven subjects.
Serological Assays and HBV DNA Assay
Serum HBsAg, HBV e antigen (HBeAg), anti-HBe, anti-HCV, and anti-HDV were measured using AxSYM MEI kits (Abbott Laboratories, North Chicago, IL). The HBV DNA level was quantified using a real-time fluorescence quantitative commercial kit (Shenzhen PG Biotech, Shenzhen, China), which has a detection limit of 1,000 HBV DNA copies/ml. Assay calibration using a standard HBV DNA was performed by the National Institute for the Control of Pharmaceutical and Biological Products, China.
HBV Genotyping
HBV genotypes were identified using a polymerase chain reaction–restriction fragment length polymorphism (PCR–RFLP) technique, as previously described [Lindh et al., 1997].
Direct Sequencing of the C Open-Reading Frame
HBV DNA was extracted from 200 µl of serum using the QIAamp DNA Blood Mini Kit (Qiagen Biotechnology Co., Ltd, Hilden, Germany). HBV sequence (671 bp) was amplified with primers P1 (5′-CATAAGAGGACTCTTGGACT-3′) and P2 (5′-TGATAAGATAGGGGCATTTG-3′). If any sample failed to amplify, the PCR was repeated using primers P4 (5′-TTTTTCACCTCTGCCTAATC-3′) and P2. A second-round semi-nested PCR used primers P1 or P4 and P3 (5′-CCTGAGTGCTGTATGGTGAGG-3′) to produce either a 416 bp or a 218 bp amplimer that both contained G1896 and the sequence of the core 18–27 peptide. The core promoter sequence was only contained in the 416 bp sequence. The amplification protocol was 1 cycle of 95°C for 2 min followed by 30 cycles of 94°C for 30 sec, 55°C for 30 sec, and 72°C for 40 sec. The PCR products were directly sequenced using either the P1 or the P4 primer on an ABI 3730 sequencer. The chromatograms were read with Chromas 2.23 (Technelysium, Tewantin, Australia), and classified as being V27, I27 or a mixture of V27 and I27 (I/V27).
Cloning and Sequencing of HBcAg18–27 Encoding Region
The second-round PCR products containing the HBcAg18–27 coding region were ligated into the pGEM-T vector (Promega, Madison, WI) and used to transform E. coli (DH5α). A minimum of six inserts of the correct size from each ligation were sequenced from the M13 primer.
HLA-A2 Typing
Peripheral blood mononuclear cells (PBMC) were isolated from 5 ml of heparinized blood by Ficoll/Hypaque density gradient centrifugation. PBMC (5 × 105) were stained with PE-conjugated mouse anti-human HLA-A2 antibody or PE-conjugated mouse IgG-isotype control antibody (BD Pharmingen, San Diego, CA) and incubated for 10 min at room temperature. The stained cells were washed twice with phosphate-buffered saline (PBS), fixed with 0.5% paraformaldehyde and analyzed on a FACS Canto II (BD Biosciences, San Jose, CA) flow cytometer.
In Vitro Generation and Analysis of Core 18–27-Specific CTL Lines
Core 18–27 peptide-specific CTL lines were generated from the PBMC of each HLA-A*02 positive patient using a modification of the method of Maini et al. [2000]. On day 1, 5 × 106/ml PBMC in 200 µl complete medium were cultured in triplicate in a 96-well U-bottomed plate in the presence of 25 ng/ml recombinant human interleukin-7 (IL-7) and 10 µg/ml of either the FLPSDFFPSV or FLPSDFFPSI peptides (Pepower Biotechnology Co., Ltd, Guangzhou, China). Cultures were carried out at 37°C in a humidified 5% CO2 incubator. Recombinant human IL-2 (50 U/ml) was added on days 3, 5, 7, 9, and 11. On day 13, cells were harvested, washed in PBS, and incubated for 10 min at 22°C with PE-conjugated HLA pentamers containing either the FLPSDFFPSV or FLPSDFFPSI peptides (Proimmune, Oxford, UK). The PBMC were then incubated with APC-conjugated anti-CD8 (BD Pharmingen) for 30 min at 4°C. The stained cells were fixed with 0.5% paraformaldehyde and analyzed on a FACS Canto II (BD) flow cytometer.
Statistical Analyses
Numerical data are summarized as the mean ± the standard deviation. Comparisons of numerical data between groups were conducted with a Kruskal–Wallis test. Comparisons of frequency data between groups were conducted with a Fisher's exact test. Statistical calculations were performed using SAS (SAS Institute, Inc., Cary, NC).
RESULTS
Association Between the Severity of CHB and the Prevalence of V27
Figure 1 shows that the prevalence of V27 detected by direct sequencing was significantly (P = 0.02) higher in the patients with severe liver inflammation (18/77, 23.4%) than in the patients with moderate liver inflammation (3/44, 6.8%). The difference in the prevalence of V27 between the ACLF (10/39, 25.6%) and moderate CHB groups was also significant (P = 0.02), and the difference between the SHB (8/38, 21.1%) and moderate CHB groups approached significance (P = 0.06). There was no difference in the prevalence of V27 between the SHB and ACLF groups. The prevalence of V27 was also significantly higher (P = 0.006) in the severe CHB group than in the combined group of subjects with inactive disease (2/45, 4.4%). The prevalence of V27 in the moderate CHB group and the inactive disease group was similar. The difference in V27 prevalence between the moderate and severe liver inflammation groups was not due to a difference in distribution of HBV genotypes between the groups, as there was a higher proportion of genotype B subjects in the severe inflammation group (Table I, P = 0.03), and V27 was less common in genotype B (9/105) than genotype C (12/53) patients (Table II, P = 0.02). There was no influence of V/I27 status on patient mortality in the ACLF group, in which 18 of 39 subjects died (Table II, P = 0.46). There was no influence of V/I27 status on HBV DNA levels (data not shown).
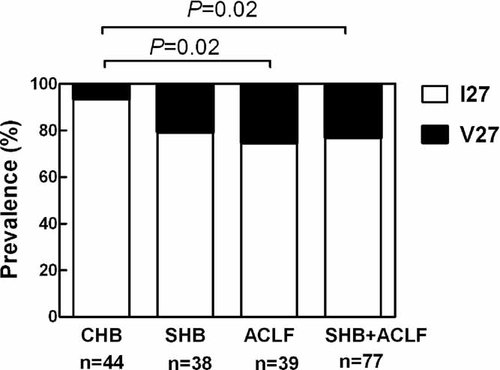
The frequency of valine at amino acid 27 of the HBV core gene in HBV genomes extracted from the serum of subjects with moderate-severity chronic hepatitis B (CHB), severe chronic hepatitis B (SHB), and acute-on-chronic liver failure (ACLF). The frequency in the combined SHB and ACLF groups is also shown as these two groups may share the same pathogenesis.
| Genotype B | Genotype C | Survived | Died | |
|---|---|---|---|---|
| I27 | 96 | 41 | 17 | 12 |
| V27 | 9 | 12 | 4 | 6 |
Influence of HLA-A*02 on Mutations at Position 27 of the HBV Core Gene
Figure 2 shows the protocol for investigating the influence of the HLA-A*02 allele on mutations at position 27 of the HBV core gene. There was no association between HLA-A*02 status and the presence (n = 15) or absence (n = 22) of V27 (P = 0.73) in patients with severe liver inflammation at presentation. Follow-up data were obtained on 30 patients, who were all on antiviral therapy, and HBV DNA was extracted from the serum of 28 of these. V27 was detected in HBV DNA from 10 of these 28 patients by direct sequencing, and the HBV DNA from the other 18 contained only I27. Figure 3A shows follow-up data from the 10 patients with V27 at baseline, and compares the changes in V/I27 status in subjects who were HLA-A*02 positive and negative. V27 was not detected in any of the five HLA-A*02 positive patients at follow-up, but could still be detected in all five HLA-A*02 negative patients (5/0 vs. 0/5, P = 0.008). An alternative analysis is that I27 occurred in all five HLA-A*02 positive patients at follow-up, but in only one HLA-A*02 negative patient (5/0 vs. 1/4, P = 0.05). Sequencing of HBV clones (Fig. 3B) from the five HLA-A*02 positive patients confirms the results of direct sequencing, in that the frequency of I27 clones has markedly increased in all patients. There was no change in the V/I27 status of any of the 18 patients who were I27 positive at baseline, as assessed by direct sequencing (data not shown).
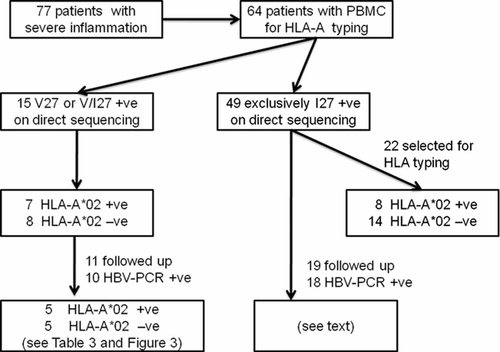
A flow chart to show how patient groups were obtained for the study of the association between HLA-A*02 and I/V27 status, and the study of the influence of HLA-A*02 on the reversion of V27 to wild-type I27.
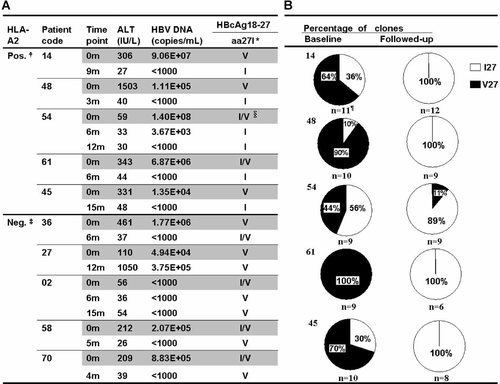
A: Comparison of the rate of reversion of V27 to I27 in groups of subjects with severe liver inflammation who were either HLA-A*02 positive or HLA-A*02 negative. The data were obtained by direct sequencing of PCR products. B: Sequencing of cloned core gene amplimers in the five HLA-A*02 positive subjects confirms the high rate of reversion of V27 to I27 found by direct sequencing.
Pentamer Staining of HBcAg18–27 Epitope-Specific CD8+ Memory Cell
Stored PBMC for analysis of CD8+ T cells recognizing either the FLPSDFFPSV (V27) or the FLPSDFFPSI (I27) peptides were available from two HLA-A*02 positive subjects and one HLA-A*02 negative subject (Table III). They all had a V27 positive virus at baseline. CD8+ T cells recognizing these peptides were not detected in the HLA-A*02 negative subject 42, whose virus remained V27 positive at follow-up. There was a higher percentage of CD8+ T cells recognizing the V27 peptide relative to the I27 peptide in both HLA-A*02 positive subjects. The HBV in both these subjects converted from V27 to I27 during follow-up.
| ID | HLA-A*02 | HBV V27I status | Peptide specific pentamer | ||
|---|---|---|---|---|---|
| Baseline | Follow-up | V27 (%) | I27 (%) | ||
| 30 | Pos | V27 | I27 | 3.74 | 0.43 |
| 54 | Pos | V27 | I27 | 0.9 | 0.07 |
| 42 | Neg | V27 | V27 | 0.05 | 0.02 |
DISCUSSION
The causes and mechanisms of the severe flares of liver inflammation that occur in patients with chronic hepatitis B are poorly understood. Part of the problem is that liver biopsy tissue cannot be obtained from these patients for direct studies of the immune cells involved, and thus indirect data are needed to test hypotheses. There is evidence that a high frequency of amino acid changes in the HBV core gene is associated with severe exacerbations of both chronic hepatitis B [Ehata et al., 1993] and acute fulminant hepatitis B [Gunther, 2006]. However there were no significant associations with specific amino acids in either these or other [Liu et al., 2003] studies. It is commonly believed that severe flares of chronic hepatitis B are caused by CD8+ T cells responding to peptide epitopes presented by HLA class I on the surface of infected hepatocytes [Liaw, 2003; Sarin et al., 2009], but the evidence for this is weak. However, this is an important hypothesis, because therapeutic vaccines designed to suppress HBV replication in chronic hepatitis B patients are likely to require stimulation of peptide-specific CD8+ T cell activity. Further effort to develop safe vaccines needs either evidence that CD8+ T cells are not involved in severe flares, or evidence that the repertoire of peptide epitopes that precipitate the flares is different to the repertoire that suppresses HBV replication through the release of interferon-gamma [Guidotti, 2002].
This study provides data that is consistent with the possibility that CD8+ T cells responding to the HBV core 18–27 peptide [Bertoletti et al., 1994] containing a valine at position 27 contribute to severe hepatitis in HLA-A*02 positive people with chronic hepatitis B. The finding of a high frequency of valine at position 27 of the core gene in patients with severe liver inflammation is consistent with the hypothesis that valine plays a role in the pathogenesis of severe disease. The additional finding that this valine converts to isoleucine in all HLA-A*02 positive patients during follow-up supports the hypothesis that there is positive selection pressure on this amino acid, and is consistent with the finding of a high frequency of changes in amino acid sequence following severe exacerbation of hepatitis B noted by Liu et al. [2003]. Since the V27 peptide is a known CD8+ T cell epitope [Bertoletti et al., 1994], it should have been possible to identify the CD8+ T cells in PBMC from these patients using pentamer technology. Although an assay to detect these cells was established in a small number of subjects, this was not sufficient to fully test the hypothesis.
It should be noted that the majority of chronic hepatitis B patients with severe liver inflammation in this study did not have valine at position 27 of their HBV core gene. This raises the issue of whether the CD8+ T cell response to the V27 peptide is the only CD8-related mechanism for severe hepatitis, or whether severe hepatitis is commonly caused by CD8+ T cell responses to viral peptides that have not yet been identified. This is a significant issue from the point of view of designing a therapeutic vaccine, since leaving a V27 peptide out of a vaccine would solve the safety problem if this was a unique mechanism. However, if there are a large number of peptides with the potential to precipitate severe flares, then it may be difficult to design a therapeutic vaccine that excludes them all.
A solution to this problem could be found if all the CD8+ T cell epitopes that cause severe flares arose within patients as a result of genetic drift. This is a process in which a non-synonymous mutation arises in an HBV genome as a result of a random HBV polymerase or cellular RNA polymerase error, or as a result of G-A hypermutation [Gunther, 2006]. The mutant may then increase in frequency in the overall viral population as a result of random selection processes. For example, 21% of the core gene amino acids (38 out of 183) in subjects with an inactive, genotype C, HBeAg-negative chronic HBV infection are under neutral selection [Abbott et al., 2010] and this includes I27 (W.G.H. Abbott, unpublished work). Neutrally selected amino acids are also common in the S, P, and X open-reading frames (William Abbott, manuscript in preparation). Thus there are a large number of potential CD8+ T cell epitopes evolving in chronic hepatitis B patients as a result of random processes. Some of these may bind to HLA class I and be seen by the immune system as a novel, non-self epitope, producing, in conjunction with danger signals from the already inflamed liver, a vigorous CTL response. If this hypothesis is correct, then therapeutic vaccines based on wild-type peptide sequences might still be feasible [Tan et al., 2010].
The alternative hypothesis is that some people in China are infected by a genotype B or C virus that already has a valine at position 27 of the core gene. The CD8+ T cells that recognized the core 18–27 peptide with V27 would presumably be anergized or exhausted [Reignat et al., 2002] at the time the chronic HBV infection was established, and then re-activated at a later time to cause an acute flare. These data do not distinguish between these two hypotheses about the origin of V27.
In summary, an association between the presence of valine at position 27 of the HBV core gene and severe exacerbations of chronic hepatitis B has been identified. Although previous studies have shown a high prevalence of core gene mutations in these patients [Ehata et al., 1993; Liu et al., 2003], this is the first time that SHB has been associated with mutation at a specific amino acid. At follow-up, V27 reverted to the genotype B/C wild-type I27 in all HLA-A*02 positive but not HLA-A*02 negative subjects, suggesting that there was positive selection pressure from CD8+ T cells recognizing the known core 18–27 peptide epitope [Bertoletti et al., 1994], FLPSDFFPSV. These data support a role for CD8+ T cells in the pathogenesis of some severe exacerbations of chronic hepatitis B.




