Significance of lung nodules detected on chest CT among adult Aboriginal Australians – a retrospective descriptive study
Abstract
Introduction
There are limited data on chest computed tomography (CT) findings in the assessment of lung nodules among adult Aboriginal Australians. In this retrospective study, we assessed lung nodules among a group of adult Aboriginal Australians in the Northern Territory of Australia.
Methods
Patients who underwent at least two chest CT scans between 2012 and 2020 among those referred to undergo lung function testing (spirometry) were included. Chest CT scans were assessed for the number, location, size and morphological characteristics of lung nodules.
Results
Of the 402 chest CTs assessed, 75 patients (18.7%) had lung nodules, and 57 patients were included in the final analysis with at least two CT scans available for assessment over a median follow-up of 87 weeks. Most patients (68%) were women, with a median age of 58 years and smoking history in 83%. The majority recorded only a single nodule 43 (74%). Six patients (10%) were diagnosed with malignancy, five with primary lung cancer and one with metastatic thyroid cancer. Of the 51 (90%) patients assessed to be benign, 64 nodules were identified, of which 25 (39%) resolved, 38 (59%) remained stable and one (1.8%) enlarged on follow-up. Nodules among patients with malignancy were typically initially larger and enlarged over time, had spiculated margins and were solid, showing no specific lobar predilection.
Conclusions
Most lung nodules in Aboriginal Australians are likely to be benign. However, a proportion could be malignant. Further prospective studies are required for prognostication and monitoring of lung nodules in this population.
Introduction
Adult Aboriginal Australians are reported to have a higher prevalence of chronic respiratory diseases than non-Aboriginal Australians, with asthma, bronchiectasis and chronic obstructive pulmonary disease (COPD) being the most common respiratory disorders.1, 2 Apart from chronic respiratory diseases, the incidence of lung cancer is also reported to be higher in the Aboriginal than the non-Aboriginal population. Moreover, lung cancer is reported to be the most commonly diagnosed cancer and the leading cause of cancer-related deaths among Aboriginal Australians.3-6
Chest computed tomography (CT) scan is fundamental in the accurate diagnosis of several pulmonary conditions.7 Lung nodules are a common radiological abnormality encountered in day-to-day clinical practice, and chest CT is more sensitive than chest X-ray in differentiating which are benign versus malignant.8 Characterisation of lung nodules as benign or malignant is paramount, as early therapeutic interventions for lung malignancy have favourable prognosis while for those nodules that are indeterminate, short- or long-term monitoring is necessary.9 In this vein, based on clinical parameters, such as age and smoking history, predictive models, protocols and guidelines have been developed for estimation of the probability that a lung nodule could be benign or malignant.10-12 However, it is imperative to note that the majority of these predictive models and guidelines that are currently developed are based on studies among non-Indigenous global populations.
Despite evidence in the literature to suggest Aboriginal Australians have a higher prevalence of chronic respiratory conditions, including lung malignancy, literature detailing chest radiology is sparse and is largely limited to chest X-ray data dating back to the 1980s.13 This is not wholly surprising though, as access to chest CT is limited in remote areas where the majority of the population is Aboriginal Australian, more specifically in the Northern Territory (NT) of Australia.14, 15 Furthermore, previous reports have indicated that there are significant barriers in facilitating care for Aboriginal patients with cancer.16
Nevertheless, currently there appears to be contrasting information. On one hand, the literature portrays an increased incidence and worse outcomes for Aboriginal Australians with lung cancer,3-6 while on the other hand, there is a fundamental lack of basic chest CT scan data for the adult Aboriginal population.13 This raises several important questions: (i) Is the geographical isolation and impaired access to investigative modalities such as CT responsible for lung cancer being diagnosed at advanced stages, giving rise to worse overall outcomes or (ii) is it due to a sparsity of chest CT data among Aboriginal Australians, which is driving delayed diagnosis and poorer sequalae or (iii) is it due to adopting guidelines, protocols and predictive models that are established on studies predominantly based on non-Indigenous populations. Due to a lack of published literature and guidelines about the clinical implications for Aboriginal patients demonstrating lung nodules on chest CT scans, it is important to investigate the clinical significance and trajectory of lung nodules detected among Aboriginal patients. Therefore, the aim of this study is to assess the significance of lung nodules detected among a group of Aboriginal patients undergoing a chest CT in the Top End Health Services (TEHS) region of the NT of Australia.
Methods
Setting
This retrospective study was conducted at the respiratory and medical imaging service based at the Royal Darwin Hospital (RDH), the major tertiary care university affiliated teaching hospital for the TEHS, NT region of Australia.
Study participants and ethical approval
This study is a part of our larger project examining lung function data, including chest radiology among adult Aboriginal Australians in the TEHS region of the NT of Australia,17 and was approved by the Human Research Ethics Committee (HREC), Health Research Governance committee and Menzies School of Health Research of the TEHS, NT (Reference: HREC 2019-3445). The study participants included in the study are a subset of patients who were identified to have lung nodules from our previous published report on chest CT findings among the adult Aboriginal patients between 2012 and 2020.18 The authors acknowledge the rights of the Indigenous people involved in this study, and as such, the study was conducted and reported according to strengthening and reporting of health research involving Indigenous people, including consultation with local institute Aboriginal Australian representative.19 Further details regarding setting and study participants are available from our previous reports.17, 18
Assessment of lung nodules on chest CT
Digital images of chest CT scans of the included study participants were initially reported by a senior radiologist based at the RDH and identified to have parenchymal lung nodules. All available digital images were further assessed among those patients reported to demonstrate lung nodules by the study author for the following parameters: (i) confirm the presence of lung nodules, (ii) location, (iii) number, (iv) size, (v) morphology and (vi) concurrent pulmonary abnormalities.
Further analysis was undertaken if the study participants had multiple CT reports available to document any interval change in lung nodule sizes (earliest to most recent CT), alongside any associated pulmonary or pleural pathology, in particular for a confirmed diagnosis of lung malignancy. The interval duration between the first and the last available CT was also recorded. Lung nodules were determined to be malignant, if malignancy was confirmed on biopsy/histopathology or consensus opinion during a multidisciplinary lung cancer meeting. Lung nodules were considered to be benign or non-malignant, if the nodules decreased in size or resolved on follow-up CT scans or review of available and relevant patient's clinical records was indicative of non-malignant causes.
Statistical analysis
Patient demographic and clinical characteristics and lung nodule morphology were reported as median [interquartile range (IQR)] for continuous or number (%) for categorical parameters. Lung nodules were defined based upon their change between first and subsequent CT scans, categorised as stable [no or minor size change (combined axis size change of ≤1 mm)], resolved [disappeared by second CT or significant size reduction (combined axis size change of ≤5 mm)], enlarged [significant size increase (combined axis size change of ≥5 mm)] or new (new nodules appearing on second CT scan). All data were analysed in STATA IC 15 (StataCorp, TX, USA), and alpha was set to 0.05 throughout.
Results
Study participants
Of the 402 patients with a chest CT within the study period, 75 patients were identified to have lung nodules and 57 patients had at least two CT scans available to assess progress during the study window. Of the 57 patients, the majority were female (68%) and remote residents (73%), with a median age of 58 years [IQR 50, 65] and a median of 87 weeks (IQR 27, 164) between the two CT scans. A history of smoking was common (83%) and only one (1.8%) patient did not record any comorbidities, with the majority (n = 35, 61.4%) reporting at least three comorbidities, of which COPD was the most common (64.9%) (Table 1). Lung function parameters (52/57 patients had acceptable spirometry results) demonstrated significant impairment, with a median forced vital capacity (FVC) of 61% predicted (IQR 48, 74), a median forced expiratory volume in 1 second (FEV1) of 54.5% predicted (IQR 38, 68) and an FEV1/FVC of 0.7 (0.6, 0.8). A significant proportion of patients demonstrated presence of concurrent chest CT abnormalities (Table 2).
| Demographics and medical comorbidities | Total (n %) (n = 57) |
|---|---|
| Female | 39 (68.4%) |
| Age (at first CT) | 57.7 (49.6, 64.7) |
| Remote (ASGS 4 or 5) (n = 44) | 32 (72.7%) |
| Past smoking history | 47 (82.5%) |
| COPD | 37 (64.9%) |
| HTN | 26 (45.6%) |
| Diabetes | 24 (42.1%) |
| CAD | 21 (36.8%) |
| CKD | 19 (33.3%) |
| Bronchiectasis | 20 (35.1%) |
| Prior history of malignancya | 12 (21.1%) |
| Breast cancer | 2 (16.6%) |
| Oesophageal cancer | 1 (8.3%) |
| Lung cancer | 1 (8.3%) |
| HCC | 1 (8.3%) |
| Thyroid cancer | 1 (8.3%) |
| Duodenal cancer | 1 (8.3%) |
| Gallbladder cancer | 1 (8.3%) |
| Pancreatic cancer | 1 (8.3%) |
| Urinary bladder cancer | 1 (8.3%) |
| Vulval SCC | 1 (8.3%) |
| Anal carcinoma | 1 (8.3%) |
| TB | 5 (8.8%) |
| Liver cirrhosis | 3 (5.3%) |
| Deceased | 15 (26.3%) |
| Age deceased | 68.6 (56.9, 79.3) |
| FVC pre-BD (%) (n = 52) | 61 (48, 74) |
| FEV1 pre-BD (%) (n = 52) | 54.5 (38, 68) |
| FEV1/FVC (absolute) (n = 52) | 0.7 (0.6, 0.8) |
- Parameters displayed as median (IQR) or number (%).
- ASGS, Australian Statistical Geography Standard; BD, bronchodilator; CAD, coronary artery disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CT, computed tomography; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; HCC, hepatocellular carcinoma; HTN, hypertension; IQR, interquartile range; SCC, squamous cell carcinoma; TB, tuberculosis.
- a Denominator for malignancy breakdown is 12.
| Concurrent chest CT findings | (n = 57) |
|---|---|
| Emphysema/COPD | 26 (45.6%) |
| Atelectasis/collapse | 10 (17.5%) |
| Bronchiectasis | 17 (29.8%) |
| Consolidation | 10 (17.5%) |
| Bullae | 2 (3.5%) |
| Cavity | 3 (5.3%) |
| Lung mass | 1 (1.8%) |
| Pleural effusion | 0 (0%) |
- Includes findings from both the first and the subsequent chest CT scans.
- COPD, chronic obstructive pulmonary disease; CT, computed tomography.
Lung nodule data
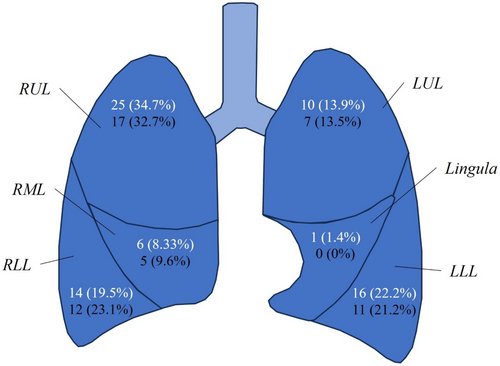
Two patients (3.5%) recorded four nodules, two patients (3.5%) recorded three nodules, 10 patients (17.5%) recorded two nodules, and the remaining 43 (74%) recorded only a single nodule, resulting in a total of 77 nodules recorded. Of these 77 nodules, 72 (93.5%) were identified on the first CT scan of which 27 (37.5%) had resolved completely by the second scan, 40 (55.6%) had remained static and five (6.9%) had enlarged, with five new nodules appearing on the follow-up CT (Table 3). Nodules which enlarged between CT scans were already larger at the first CT, with a greater proportion spiculated (80%). Of those nodules which enlarged, four (80%) were associated with malignancy, of which three (75%) were spiculated, while the one enlarged nodule not associated with malignancy was also spiculated. Of those that remained static, a greater proportion were subpleural (57.5%) and calcified (17.5%) compared to those that resolved (37% and 3.7%, respectively) or enlarged (40% and 0%, respectively). The majority of lung nodules were located in the right lung, particularly in the upper lobe (Fig. 1). Examples of lung nodules observed in the study participants are shown in Figures 2 and 3.
| Static (n = 40) | Resolved (n = 27) | Enlarged (n = 5) | New (n = 5) | |
|---|---|---|---|---|
| First CT | ||||
| Short axis (mm) | 5 (3, 6) | 5 (3, 8) | 9 (6, 10) | – |
| Long axis (mm) | 5 (4, 7) | 5 (3, 11) | 12 (10, 15) | – |
| Spiculation | 1 (2.5%) | 2 (7.4%) | 4 (80%) | – |
| Calcification | 7 (17.5%) | 1 (3.7%) | 0 (0%) | – |
| Subpleural | 23 (57.5%) | 10 (37%) | 2 (40%) | – |
| Solid | 39 (97.5%) | 24 (88.9%) | 5 (100%) | – |
| Subsolid/part solid | 1 (2.5%) | 3 (11.1%) | 0 (0%) | – |
| Second CT | ||||
| Short axis (mm) | 5 (3.5, 6.5) | – | 14 (11, 21) | 7 (6, 7) |
| Long axis (mm) | 5 (4, 7) | – | 16 (12, 21) | 10 (8, 12) |
| Spiculation | 1 (2.5%) | – | 4 (80%) | 0 (0%) |
| Calcification | 7 (17.5%) | – | 1 (20%) | 1 (20%) |
| Subpleural | 21 (52.5%) | – | 2 (40%) | 1 (20%) |
| Solid | 39 (97.5%) | – | 5 (100%) | 5 (100%) |
| Subsolid | 1 (2.5%) | – | 0 (0%) | 0 (0%) |
| Time between first and second CT (weeks) |
98 (34.5, 159.29) Min. 1.4 Max. 393 |
74.4 (36.7, 196.1) Min. 4.9 Max 473.6 |
103 (91.3, 127.3) Min. 54 Max. 265.1 |
87.7 (51.7, 87.7) Min. 36.7 Max. 127.3 |
| >12 weeks between CT scans (n (%)) | 36 (90%) | 25 (92.6%) | 5 (100%) | 5 (100%) |
| Final determination of nature | ||||
| Benign | 38 (95%) | 25 (92.6%) | 1 (20%) | 1 (20%) |
| Malignancy | 2 (5%) | 2 (7.4%) | 4 (80%) | 4 (80%) |
- Parameters displayed as median (IQR) or number (%). Determination of nature of lung nodules was considered at the patient level – therefore, although 12 nodules are marked as malignant, this refers to only six patients, three of whom had multiple nodules present.
- CT, computed tomography; IQR, interquartile range.
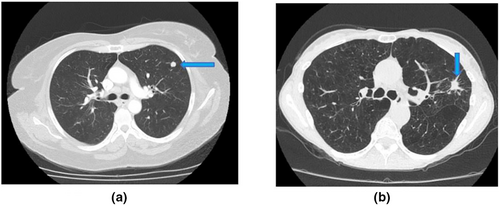
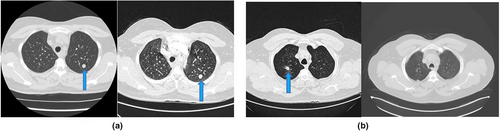
Overall, six patients were considered to have malignancy – three had only a single nodule recorded, while the other three had multiple nodules with newly developed nodules in subsequent imaging (described below). The distribution included right lower lobe, left lower lobe and right upper and left upper lobes. Of the three patients with only a single nodule – with the first patient, the nodule was sized 9 × 15 mm in the first CT and increased to 14 × 21 mm in the second CT. With the second patient, the nodule was sized 17 × 26 mm in the first CT and increased to 30 × 47 mm in the second CT. For the third patient, the nodule was sized 10 × 12 mm in the first CT and increased to 11 × 16 in the second CT. This patient also had a history of concurrent vulval cancer.
Of the three patients with malignancy and multiple/newly developed nodules, two patients had one new nodule develop and one patient had two new nodules develop. The first patient had three nodules at the first CT (3 × 3, 4 × 4 & 7 × 7 mm) the largest of which remained static to the second CT, the middle of which resolved and the smallest of which enlarged to 10 × 10 mm with the new nodule measuring 8 × 8 mm. This patient also had a history of thyroid cancer. The second patient had one nodule at the first CT (5 × 5 mm) which resolved by the second CT when the new nodule developed (sized 7 × 14 mm). The third patient had one nodule at the first CT which was sized 4 × 4 mm and remained static, with two new nodules developing by the second CT (3 × 3 & 6 × 10 mm). This patient had a history of lung cancer (right middle lobe) with Stereotactic ablative body radiotherapy conducted. The clinical characteristics of patients with benign and malignant nodules are shown in Table 4.
| Clinical parameters | Benign | Malignant | ||
|---|---|---|---|---|
| Patients (n) | 51 | 6 | ||
| Age (first CT) | 55.8 (47.9, 64.7) | 60.45 (58.8, 69.4) | ||
| Sex (female) | 33 (64.7%) | 6 (100%) | ||
| Time between first and second CT (weeks) |
84.9 (24.4, 171.9) Min. 1.4 Max. 473.6 |
95.4 (54, 127.3) Min. 51.7 Max. 265.1 |
||
| >12 weeks between CT scans | 45 (88.2%) | 6 (100%) | ||
| Chest CT | First CT | Second CT | First CT | Second CT |
| Nodules (n) | 64 | 42 | 8 | 10 |
| Short axis (mm) | 5 (3, 7) | 5 (4, 7) | 6 (4, 9.5) | 7 (3.5, 10.5) |
| Long axis (mm) | 5 (4, 9) | 5 (4, 8) | 6 (4, 13.5) | 9 (3.5, 15) |
| Spiculation | 4 (6.3%) | 3 (7.1%) | 3 (37.5%) | 3 (30%) |
| Calcification | 7 (10.9%) | 7 (16.7%) | 1 (12.5%) | 2 (20%) |
| Subpleural | 34 (53.1%) | 22 (52.4%) | 4 (50%) | 3 (30%) |
| Solid | 60 (93.8%) | 41 (97.6%) | 8 (100%) | 10 (100%) |
| Subsolid | 4 (6.2%) | 1 (2.4%) | 0 (0%) | 0 (0%) |
| Static | 38 (58.5%) | 38 (90.5%) | 2 (25%) | 2 (20%) |
| Resolvedb | 25 (38.4%) | 2 (4.8%) | 2 (25%) | 0 (0%) |
| Enlarged | 1 (1.5%) | 1 (2.4%) | 4 (50%) | 4 (40%) |
| New | – | 1 (2.4%) | – | 4 (40%) |
| Emphysemaa | 21 (41.2%) | 19 (37.3%) | 4 (66.7%) | 4 (66.7%) |
| Bronchiectasisa | 12 (23.5%) | 14 (27.5%) | 1 (16.7%) | 1 (16.7%) |
| Lymph nodesa | 9 (17.6%) | 8 (15.7%) | 3 (50%) | 2 (33.3%) |
| Consolidationa | 7 (13.7%) | 5 (9.8%) | 0 (0%) | 1 (16.7%) |
| Atelectasisa | 5 (9.8%) | 5 (9.8%) | 0 (0%) | 1 (16.7%) |
| Cavitya | 2 (3.9%) | 2 (3.9%) | 0 (0%) | 0 (0%) |
| Bullaea | 0 (0%) | 1 (2%) | 1 (16.7%) | 1 (16.7%) |
| Collapsea | 1 (2%) | 1 (2%) | 0 (0%) | 0 (0%) |
| Lung massa | 0 (0%) | 0 (0%) | 0 (0%) | 1 (16.7%) |
- a Concurrent lung fundings are listed at the person level, and thus, the denominators are 51 (benign) and 6 (malignant).
- b Two nodules among benign patients were marked as resolved yet still visible at the second CT, though reduced in size.
Discussion
To the best of the authors knowledge, this is the first study to describe the importance of lung nodules detected on chest CT scan among an Aboriginal Australian cohort from the NT of Australia. In this retrospective analysis involving adult Aboriginal Australians, lung nodules were detected in 75 out of 402 patients, yielding a lung nodule detection rate of 18.7%. However, among the 75 patients with initially detected lung nodules, only 57 (76%) underwent subsequent CT scans, of whom the majority (72.8%) resided in remote or very remote communities. The majority of patients (89.5%) had nodules which did not demonstrate any form of malignancy and three-quarters (74.5%) of those without malignancy showcased stable lung nodules, with either no change or minor changes in nodule size upon subsequent follow-up CT scan.
Traditional lung nodule management guidelines focus on individual risk assessment, encompassing factors such as nodule size, morphology, sex, age, nodule location, multiplicity, smoking history, family history of lung cancer and presence of emphysema.11 Quantitative prediction models aid in estimating malignancy probability, yet no single model prevails.20 In this study, six (10.5%) patients were diagnosed to have lung malignancy, among whom the notable observation was that the lung nodule appeared to increase in size or there was detection of new nodules on follow-up CT scans. Of these, three had biopsy-confirmed malignancies through endobronchial ultrasound or via CT-guided lung nodule biopsy. For the remaining three, a radiological lung cancer diagnosis was established during a multidisciplinary cancer meeting, given their unsuitability for biopsy or resection due to compromised physical status and due to significantly reduced lung function parameters. This illustrates the complexity surrounding the diagnosis and management of respiratory disorders due to quite advanced and complex multi-morbidity among adult Aboriginal Australians.21
During the assessment of risk factors, characteristics such as nodule size and morphology, encompassing spiculated margins and solid or subsolid nature of lung nodules, play pivotal roles in estimating malignancy risk. Moreover, lung nodule size exhibits a well-established correlation with malignancy risk, alongside spiculated margins as a recognised indicator of malignancy.22, 23 In the current study, the malignant lung nodules displayed larger initial dimensions compared to benign counterparts. Furthermore, of the total 12 nodules identified among patients diagnosed with malignancy, three (25%) exhibited spiculated margins, in comparison with four (6.3%) out of the 64 nodules identified among patients not diagnosed with malignancy, suggesting an association between margin characteristics and malignancy in this cohort. Solid lung nodules were consistently detected via CT scans in all six patients diagnosed with malignancy. Notably, subsolid lung nodules detected within this study were non-malignant, as most exhibited resolution during follow-up CT scans.
In terms of lung nodule location, lung cancers tend to manifest more frequently in the upper lobes, particularly favouring the right lung.24, 25 This pattern was affirmed as a risk factor for malignancy in the PanCan trial.22 Moreover, drawing from a prior study, the escalation of primary cancer risk aligned with an increasing nodule count from one to four, but conversely, a decreased risk was observed in patients with five or more nodules, predominantly attributed to prior granulomatous infections.26 The present study did not yield any discernible predilection between nodule location nor multiplicity for malignancy. However, with only six malignant outcomes the power to detect any such associations is limited. Overall, though, lung nodule distribution favoured the right lung with 62.5% of nodules identified at the first CT scan, and this proportion increased to 65.4% by the second CT – at both timepoints the right upper lobe had the largest number of nodules.
A widely acknowledged risk factor tied to malignancy is advancing age.27 The PanCan trial also reported female gender as a risk factor for malignant lung nodules.22 Our study mirrors this pattern, as all patients with diagnosed malignancy were female and of an older age compared to those with benign nodules. Emphysema alongside lung nodules identified on chest CT scans constitutes an independent risk factor for lung cancer.28 Notably, in the NLST trial, the emphysema-predominant COPD phenotype and escalating centrilobular emphysema severity were linked to heightened malignancy risk.29 Although the data gleaned from our study underscore a less compelling trend, four (66.7%) patients with malignant lung nodules exhibited underlying emphysema; however, given the higher prevalence of emphysema in a much younger Aboriginal population,1, 17, 18 it is unclear whether this could be considered as a true association. In a similar vein, smoking is widely regarded as the most important risk factor for lung cancer. In our study, 83% of patients reported a history of smoking, both in the malignant and in the non-malignant groups. Hence, it imposes a unique clinical conundrum in stratifying smoking as a risk factor for lung cancer in Aboriginal Australians. Nonetheless, these aforementioned findings raise a serious question of whether what is observed in non-Aboriginal populations is applicable to the adult Aboriginal Australian population. Furthermore, of concern is the lack of clear established protocols for monitoring of lung nodules among Aboriginal Australians. Previous reports have indicated that during the assessment of the probability of malignancy, approximately 20% of observed nodules/cancer decreased in size at least at some point during the follow-up period.30 In our study, we observed that the vast majority of lung nodules on follow-up CT remained static or resolved. We are uncertain at this stage if some of these nodules may also develop into malignancy, only future long-term follow-up studies will shine some light in this area. In this study, we observed that 12 (21.1%) of our study participants had an established diagnosis of other non-lung malignant diseases. It is a matter of speculation how many would have a lung metastasis secondary to their primary non-lung malignancy among those demonstrating to have stable lung nodules with long-term follow-up. It is apparent that there may be a need for differing risk stratification and chest CT monitoring guidelines among Aboriginal Australian patients demonstrating lung nodules.
This study for the first time has described the significance and characteristics of lung nodules detected on chest CT among an adult Aboriginal population in the NT of Australia. This may be considered as a stepping stone to move forwards onto future prospective studies, including not only the adult Aboriginal Australians in other Australian states and territories but also among other Indigenous populations globally. Moreover, the majority of this study cohort resided in remote and regional communities and are known to have advanced and complex lung disease.31-51 Hence due to geographical isolation and limited access to specialist health care, including easy access to chest CT scans, there is a major hurdle in the long-term monitoring of lung nodules. Therefore, robust prospective radiology data are desperately needed as done in other population settings,52 in the future to elucidate lung nodule predictive models specific to Aboriginal Australians, while considering realistic limitations of remoteness and cultural factors.
Limitations
This study's findings are specifically applicable to Aboriginal adults residing in the TEHS region of the NT in Australia. Therefore, the generalisability of the results to all Australian Aboriginal populations is limited. Furthermore, the number of patients included in the study is relatively small, and with only six patients demonstrating malignancy, there is limited ability to draw firm conclusions from any statistical modelling. Another constraint lies in the incomplete documentation within medical records, rendering the measurement and confirmation of vital variables such as pack-years of smoking and family history of lung cancer unattainable. Moreover, clinical symptoms, physical examination findings, histopathology and microbiology results were not assessed in detail in all patients, especially among those considered to have benign nodules. Furthermore, the reliance on radiological rather than biopsy-confirmed malignancy diagnoses for three of the six patients with malignant lung nodules presents an additional limitation and may significantly skew results. The absence of a standardised interval for follow-up CT scans introduces variability, as the retrospective nature of the study involved the analysis of multiple CT reports with inconsistent time intervals between the initial and final CT scans. This could impact the accuracy of the findings due to variations in follow-up duration. Finally, the exclusion of 20 patients without follow-up scans, representing a significant proportion of the study cohort, is a likely source of bias. Nonetheless, this is the first study to assess the significance of lung nodules detected on chest CT scan among Aboriginal Australian adults, hence could be considered as cornerstone for future comparative studies.
Conclusion
This study highlights that while a majority of Aboriginal Australian patients in the TEHS, NT region exhibit benign lung nodules, a subset of 10% could be indicative of malignancy. Advancing age, female gender and larger initial nodule size with spiculated margins may be risk factors for malignancy in this context. However, high background prevalence of classical risk factors such as smoking and COPD/emphysema limits the use of these risk predictors in this context until larger trials are conducted. To comprehensively characterise the underlying risk factors of malignant lung nodules, further studies are imperative. Additionally, the development of quantitative prediction models specific for Aboriginal Australians and for other Indigenous people globally necessitates consideration, utilising a larger population-based cohort for more robust insights.
Acknowledgements
We would like to thank our respiratory clinical nurse consultants, Mrs Raelene Messenger and Mrs Siji Issac from the respiratory chronic disease unit, at the RDH, including rural and remote community Aboriginal health workers, and all the RDH medical services, including radiology, pathology and patient travel division for coordinating care for Aboriginal people living in the remote and rural communities. We also would like to thank our research assistant Ms Ara Perez in the data collection. We also extend our sincere appreciation to our former Aboriginal health worker, Mr Izaak Thomas (Australian Indigenous Luritja descendent) from the respiratory chronic respiratory disease co-ordination division in supporting much needed data addressing the diagnosis and management of adult Aboriginal patients with respiratory disorders.
Clinical trial registration: Not applicable. Open access publishing facilitated by Flinders University, as part of the Wiley - Flinders University agreement via the Council of Australian University Librarians.
Funding Information
Nil to declare.
Conflict of Interest
The authors declare no conflict of interest.
Ethical Approval
This study was approved by the local Human Research Ethics Committee.
Open Research
Data Availability Statement
Research data are not shared.




