An evaluation of ankle and foot bolus in paediatric modulated arc total body irradiation (MATBI)
Abstract
Introduction
This retrospective planning study aimed to evaluate the role of bolus in achieving dose uniformity in the ankles and feet in paediatric patients undergoing Modulated Arc Total Body Irradiation (MATBI) treatment and to identify patient factors that may negate or warrant its use.
Methods
The clinically treated plans of 20 paediatric patients who received MATBI treatment utilising ankle and foot bolus (Bolus plan) were compared with two retrospectively generated plans; a plan with bolus removed and no re-optimisation (No Bolus plan), and a re-optimised plan without bolus attempting to achieve equal dosimetry to the clinical plan via monitor unit adjustment (MU plan). Descriptive statistics were used to evaluate the dose uniformity criteria of ±10% coverage of the reference dose (RD) for each subregion of the ankle and foot for the three plans. The impact of patient height, weight, and age at the time of treatment was evaluated using Spearman's correlation.
Results
Variation in doses >10% RD was minimal across the three plans, with an average D1cc difference < 0.4Gy. For the ankle and foot regions in the Bolus plans, the volume receiving at least 90% of the RD (V90) was on average > 92%. In No Bolus and MU plans, there was an average reduction of 24.5% and 23.2% V90 coverage respectively in the toes. Spearman's correlation suggests height has the strongest relationship to D1cc.
Conclusion
This study validated the continued use of ankle and foot bolus to achieve dosimetric goals for paediatric MATBI treatments, particularly V90 coverage across all heights.
Introduction
Haematological malignancies account for a large proportion of childhood cancer diagnoses in Australia in the last decade.1 One treatment offered for blood cancer includes a bone marrow transplant, involving the transfer of healthy stem cells from a donor to the recipient to stimulate bone marrow production. Total body irradiation (TBI) is a specialised radiation therapy technique that can be utilised in the preparation regimen prior to the transplant2 as it is known to effectively target sanctuary bone marrow sites where drugs cannot reach.3 The aim of TBI is to achieve ±10% of the prescribed dose to the entire body,4 to eradicate disease, create room for new stem cells and suppress the immune system to reduce the risk of rejection of the transplant cells.2, 5, 6 Historically, TBI has been performed using a variety of methods including parallel-opposed open fields at an extended source to surface distance (SSD), and multiple junctioning fields.6 More recent approaches have included three-dimensional conformal radiation therapy (3DCRT) and intensity-modulated and full volumetric modulated arc techniques. At the Radiation Oncology Princess Alexandra Hospital Raymond Terrace (ROPART) department, modulated arc total body irradiation (MATBI) was adopted to treat paediatric patients, commonly between the ages of 2 and 18 years.7, 8
One of the challenges with TBI is achieving dose homogeneity. Dose homogeneity requirements are outlined in the International Commission of Radiation Units and Measurements (ICRUs) guidelines, for external beam radiation therapy.9 ICRU Report 62 defines hotspots and clinically significant maximum dose regions as volumes of tissue outside and within the planning target volume (PTV) respectively, receiving a dose greater than 100% of the prescribed dose. Recognising that the entire patient contour is the PTV for TBI treatment, the task to reduce inhomogeneity is challenging due to changes in patient separation from head to foot, particularly in the extremities such as the hands and feet. Radiation toxicity concerns are further heightened in paediatric patients due to susceptibility to cell damage and longer-term growth considerations.10 The need to reduce late skeletal and growth complications in paediatric patients treated with radiation therapy emphasises the importance of improving dose homogeneity for the MATBI technique.
Several articles present clinical experiences and cross-observational studies on the recent implementation of the MATBI technique. As reported by Kirby et al.,11 their aim was to create a robust, inversely planned TBI method, that was comfortable and achieved better dose homogeneity than previous techniques. As discussed by Pemberton et al., 3–5° gantry angle variation is utilised to create an arc formation of 40 × 40 cm beams to treat the full length of the patient laying prone and then supine.8 This technique utilises a spoiler, situated 10–15 cm from the patient surface. This creates a spoiler electron cloud (SEC) contributing to the skin dose across the length of the patient. Whilst MATBI enables improved dose modulation with the use of approximately 40 static beams, challenges remain due to variations in patient thickness. In particular, Effeney et al. demonstrated hot spots in the ankles of each patient with MATBI without the use of a bolus.7 Some methods used to address dose variations include adjustment of beam parameters such as beam angles, jaw position, and monitor unit (MU) modification. The success of these methods can be patient-dependent and have demonstrated beneficial outcomes in thin anatomical structures such as the arms and hands, with limited improvement in the feet.11-13
With no published evidence to support its use, the use of bolus in the ankles and feet was introduced at ROPART to improve dose homogeneity for MATBI based on radiation dosimetry principles. Now that several years' worth of patients have been treated with MATBI, incorporating the use of ankle and foot bolus at ROPART, it is prudent to analyse the impact of this intervention and its efficacy. The use of bolus with MATBI treatment requires additional resources and increases the simulation and treatment times for paediatric patients. Therefore, the primary aim of this planning study was to evaluate the efficacy of ankle, toe, and underfoot bolus in achieving dose uniformity and prescription goals in paediatric patients undergoing TBI treatment using the MATBI technique. The project also aimed to determine if there are any patient-specific factors that may be used as indicators for its use.
Methods
Patient selection
Ethics approval was granted (Metro South HREC/2021/QMS/80657, QUT UHREC 5473—HE26) for the retrospective use of data from paediatric patients treated with MATBI as of August 2021 at Radiation Oncology Princess Alexandra Hospital Raymond Terrace (ROPART). Of the 47 patients treated to date with this technique, the 20 most recently treated eligible patients were used. Eligibility criteria required patients to be 2–18 years old, treated with the standard dose fractionation of 12Gy in 6 fractions and with 1 cm thick ankle and foot bolus. The patients included were categorised into the following age brackets; under 5 years, 5–12 years, and over 12 years, with a minimum of three patients in each group to provide sufficient patient dimension variation. Patient demographic information including height, weight, and age at the time of treatment were collected from the MOSAIQ Oncology Management System (Elekta, Stockholm, Sweden), (Versions 2.4.1 & 2.6.0).
Bolus specifications
The bolus used for all patients in this study was made prior to the patients' computed tomography (CT) planning session. This involved a 1 cm thick bolus (a flexible commercially available, silicone-based bolus) placed on the soles of the feet and anterior of the foot including the toes (Fig. 1A). To create this piece, first staff measured the patient's feet to determine the distance from the toes to the instep, and a tracing of their footprint made, ensuring the bolus was wide enough to be joined at the medial and lateral edges of each foot. Another strip approximately 10 cm wide was wrapped around the ankles with the inferior aspect covering 1 cm superior of the medial malleolus, all held in place using a tubular bandage to create a bolus ‘sock’ (Fig. 1B). These bolus components are hereafter referred collectively as ankle and foot bolus. Figure 1C and D demonstrates that patient-specific variation in bolus dimensions occurred when factoring in foot size and flex to facilitate reproducible positioning for each treatment fraction.
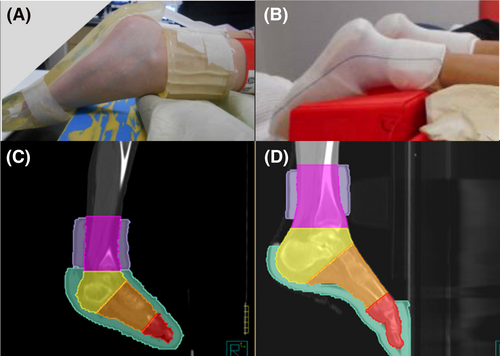
Bolus and regions of the foot contour creation
The original clinical treatment plan was restored for each patient into the treatment planning system Pinnacle3, Versions 14 and 16 (Philips Healthcare, Fitchburg, WI, USA). For these plans, the planning CT scan of each patient scanned in the prone position and with ankle and foot bolus in place was used for dose calculation as per department protocol.7 For this study, a series of contours were adapted or created for the bolus, and in the foot and ankle regions. A threshold-based auto-contouring tool was then used to create a new region of interest incorporating both the patient's external skin surface and the bolus. To create a separate contour of the bolus only, the region of interest (ROI) expansion and contraction tool, excluding the body contour was used. To facilitate the removal of the bolus, to be described in more detail in the following section, a uniform 0.5 cm expansion of the bolus, excluding the patient body contour, was created to ensure all bolus voxels were included in the density override. Manual visual assessment, fine adjustment, and contour cleaning occurred in each stage of the contour creation.
The foot and ankle regions were further divided into a total of four anatomical subregions: the ankle, heel, midfoot, and toes (Fig. 1C and D). Using the junctions and names in Table 1, the Pinnacle3 Part contouring tool was used to create these four dose reporting structures. To avoid any discrepancy in region junctions due to varying foot position or flexion, right and left ROI for the ankle, heel, midfoot and toes were created separately and combined to create the four subregions. All contours were reviewed by a senior radiation therapist for consistency and accuracy.
| ROI | Proximal border | Distal border |
|---|---|---|
| Ankle | Proximal ankle bolus | Joint between tibia and talus bones |
| Heel | Joint between tibia and talus bones | Most distal aspect of the calcaneus bone |
| Midfoot | Most distal aspect of the calcaneus bone | Joint between 5th metatarsal and 5th proximal phalanx |
| Toes | Joint between 5th metatarsal and 5th proximal phalanx | Distal toes |
- Abbreviation: ROI, region of interest.
Retrospective treatment plan formulation
Three plans were developed for each patient: Bolus, No Bolus, and Monitor Unit (MU). The Bolus plan was the original plan unaltered, representing the delivered dosimetry with ankle and foot bolus applied. The No Bolus plan represented the dosimetry if the bolus was absent. To create this plan, the original plan was copied, and the relative electron density of the expanded bolus structure was then overridden to equal zero. The dose was recalculated for all beams. The third plan, MU, was created, to determine if clinically comparable dosimetry to the original plan could be achieved after removal of bolus, by adjusting the MU. The MU plan was created by copying the No Bolus plan. The beam display parameter features were used to determine which of the anterior and posterior beams contribute dose to the ankle and foot region. Initially, the inferior beams traversing below the knees only were adjusted first, to avoid dose alteration to superior organs. Relevant beams were adjusted by adding or subtracting MU from the original MU in 2–3 MU increments using a visual assessment of dosimetry. The main considerations included a visual comparison of the isodose distribution to the original plan and improving dose homogeneity via monitoring of dose metrics and hotspot location.
The decision to terminate the MU adjustment process of the inferior beams was made when no further improvement was observed in dosimetry. Criteria on when this decision was made included larger hotspots and cold spots, increasing heterogeneity and the mean, minimum and maximum dose of each subregion. If dosimetry was unable to be improved, minor adjustments of superior beams were also considered. For example, subtracting 2–5% of the original MU in a beam where the inferior penumbra edge passed through the hotspot of the ankle. When making fine adjustments to the superior beams, a visual assessment of the dosimetry in the pelvis was undertaken to ensure no significant change in dosimetry.
Dose metrics used for analysis were decided on clinical relevance determined by TBI specialist Radiation Oncologists and Advanced Radiation Therapists at ROPART. This included the minimum dose to the maximally irradiated one cubic centimetre (D1cc), and volumetric metrics representing the volume of the regions receiving at least 100% of the prescribed reference dose of 12Gy (V100), as well as ±10 and 15% of the prescribed 12Gy dose, V85, V90, V110, and V115, respectively. These dose metrics were recorded for all three plans per patient.
Plan comparison and statistical analysis
Dose metrics and patient factor data were imported into the R statistical software (https://www.r-project.org/) for plan comparison and analysis of the impact of patient-specific factors. Descriptive statistics including mean, median, interquartile range, standard deviation, maximum and minimum of each dose metric were calculated by plan type, and ankle and foot subregion. Box plots provided a visual comparison of each plan type (Bolus, No Bolus, and MU), grouped by subregion and dose metric.
To determine the relationship between patient-specific factors and the impact of bolus on dose variation, scatter plots, and Spearman's correlation tests were performed. Each scatter plot represented a dose metric against a patient factor (height, weight, or age at the time of radiation treatment), for each subregion, grouped by plan type, with a line of best fit added for each plan type. For example, dose to 1cm3 of the ankle region against patient height, grouped by plan type. A corresponding Spearman's correlation test was performed for each dose metric, subregion and plan type and averaged across all regions of the foot for exploratory analysis.
Results
Bolus versus No Bolus plans
The dose metrics in each region of the foot for each plan type are shown in Table 2. Dose metrics used to assess higher doses within each subregion included D1cc, indicating the minimum dose to the maximally irradiated 1.0 cm3 and V110, representing the relative volume of each region receiving +10% of the prescribed dose. In comparison to Bolus plans, the No Bolus plans had a median D1cc dose increase, averaged across all patients of 0.04Gy in the ankle region, 0.3Gy in the heels, 0.13Gy in the midfoot and 0.06Gy in the toes. The median volume receiving at least 13.2Gy (V110) increased on an average by 5.4% across all patients and subregions when bolus was removed specifically, 4.2% in the ankles, 13.5% in the heels, 6.0% in the midfoot, and decreased by 2.2% in the toes.
| Dose metric | Region | Plan type | Median (% for Vx: Gy for D1cc) | DiffBolus (% for Vx: Gy for D1cc) | Dose metric | Region | Plan type | Median (% for Vx: Gy for D1cc) | DiffBolus (% for Vx: Gy for D1cc) |
|---|---|---|---|---|---|---|---|---|---|
| V85 | Ankles | Bolus | 98.33 | V90 | Ankles | Bolus | 97.82 | ||
| No Bolus | 89.15 | −9.19 | No Bolus | 86.50 | −11.32 | ||||
| MU | 89.37 | −8.96 | MU | 86.80 | −11.02 | ||||
| Heel | Bolus | 95.74 | Heel | Bolus | 94.07 | ||||
| No Bolus | 90.41 | −5.34 | No Bolus | 87.98 | −6.09 | ||||
| MU | 90.62 | −5.13 | MU | 88.28 | −5.80 | ||||
| Midfoot | Bolus | 96.29 | Midfoot | Bolus | 95.24 | ||||
| No Bolus | 89.57 | −6.72 | No Bolus | 87.21 | −8.04 | ||||
| MU | 89.57 | −6.72 | MU | 87.26 | −7.98 | ||||
| Toes | Bolus | 97.08 | Toes | Bolus | 94 | ||||
| No Bolus | 74.72 | −22.36 | No Bolus | 69.49 | −24.51 | ||||
| MU | 75.33 | −21.75 | MU | 70.79 | −23.21 | ||||
| V100 | Ankles | Bolus | 69.11 | V110 | Ankles | Bolus | 6.56 | ||
| No Bolus | 62.89 | −6.22 | No Bolus | 10.79 | 4.24 | ||||
| MU | 66.27 | −2.84 | MU | 13.95 | 7.40 | ||||
| Heel | Bolus | 70.90 | Heel | Bolus | 7.55 | ||||
| No Bolus | 74.53 | 3.64 | No Bolus | 21 | 13.45 | ||||
| MU | 76.87 | 5.97 | MU | 20.98 | 13.43 | ||||
| Midfoot | Bolus | 72.27 | Midfoot | Bolus | 13.24 | ||||
| No Bolus | 72.24 | −0.03 | No Bolus | 19.19 | 5.95 | ||||
| MU | 74.03 | 1.77 | MU | 21.09 | 7.85 | ||||
| Toes | Bolus | 39.62 | Toes | Bolus | 11.33 | ||||
| No Bolus | 43.01 | 3.39 | No Bolus | 9.13 | −2.2 | ||||
| MU | 47.79 | 8.17 | MU | 10.45 | −0.88 | ||||
| V115 | Ankles | Bolus | 0.16 | D1cc | Ankles | Bolus | 13.84 | ||
| No Bolus | 0.37 | 0.21 | No Bolus | 13.88 | 0.04 | ||||
| MU | 0.51 | 0.35 | MU | 13.93 | 0.09 | ||||
| Heel | Bolus | 1.41 | Heel | Bolus | 14.2 | ||||
| No Bolus | 5.65 | 4.24 | No Bolus | 14.50 | 0.30 | ||||
| MU | 6.36 | 4.95 | MU | 14.56 | 0.36 | ||||
| Midfoot | Bolus | 2.26 | Midfoot | Bolus | 14.41 | ||||
| No Bolus | 4.72 | 2.46 | No Bolus | 14.54 | 0.13 | ||||
| MU | 5.34 | 3.08 | MU | 14.63 | 0.22 | ||||
| Toes | Bolus | 3.75 | Toes | Bolus | 14.33 | ||||
| No Bolus | 3.57 | −0.18 | No Bolus | 14.39 | 0.06 | ||||
| MU | 4.16 | 0.42 | MU | 14.45 | 0.12 |
- Abbreviations: V85, relative volume (%) receiving at least 85% of the reference dose; V90, relative volume (%) receiving at least 90% of the reference dose; V100, relative volume (%) receiving at least 100% of the reference dose; V110, relative volume (%) receiving at least 110% of the reference dose; V115, relative volume (%) receiving at least 115% of the reference dose; D1cc, minimum dose to the maximally irradiated 1.0 cm3; Bolus, clinically treated plan utilising ankle and foot bolus; No Bolus, retrospective plan with removal of bolus; MU, retrospective plan with the removal of bolus and adjustment of monitor units in attempt to achieve similar dosimetry to ‘Bolus’ plan; DiffBolus, difference from Bolus plan median values; Median and DiffBolus units are percentage volume (%) for Vx metrics and dose (Gy) for D1cc;
Boxplots for the volumetric dose variation can be seen in Figure 2. The median coverage of each subregion receiving at least 10.8Gy (V90) decreased by 11.3% in the ankle region, 6.1% in the heels, 8.0% in the midfoot, and 24.5% in the toes when the bolus was removed. Reduction in volume of each subregion receiving at least 12Gy (V100) decreased on average by 6.2% in the ankles, increased by 3.6% in the heels, decreased by 0.03% in the midfoot and increased by 3.4% in the toes.
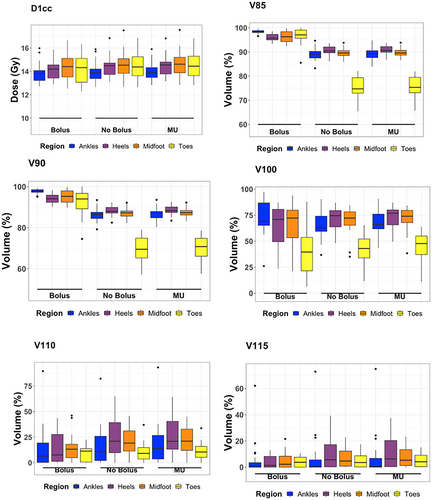
Dose metrics demonstrating dose variation outside the primary aim of ±10% of the prescribed dose include V85 and V115, representing the relative volume received −15% and +15% of the prescribed dose. The median volume receiving at least 10.2Gy (V85) decreased by an average of 10.9% coverage when the bolus was removed. Specifically, a decrease of 9.2% in the ankles, 5.3% in the heels, 6.7% in the midfoot, and 22.4% in the toes. The median volume receiving at least 13.8Gy (V115) increased by an average of 1.7% coverage when bolus was removed, 0.2% in the ankles, 4.2% in the heels, 2.5% in the midfoot, and decreased by 0.2% in the toes.
Bolus versus MU adjustment plans
Utilising MU adjustment to improve dosimetry for each subregion without bolus, achieved a median V90 coverage 12% less than the Bolus plan on average, 11% in the ankles, 5.8% in the heels, 8% in the midfoot and 23.2% in the toes. Slightly superior V100 statistics were achieved in the MU plans, with improved coverage by 6% in the heels, 1.8% in the midfoot, 8.2% in the toes, and decreased coverage in the ankles by 2.8% compared to the Bolus plan. V110 coverage also increased in MU plans for the ankles, heels, and midfoot, by 7.4%, 13.4%, and 7.9%, respectively, and decreased by 0.9% in the toes. Comparable D1cc dose was also achieved in the MU plans compared to the Bolus plans, achieving a 0.2Gy higher median D1cc dose on average, 0.09Gy in the ankles, 0.36Gy in the heels, 0.22Gy in the midfoot, and 0.12Gy in the toes.
Patient-specific predictors
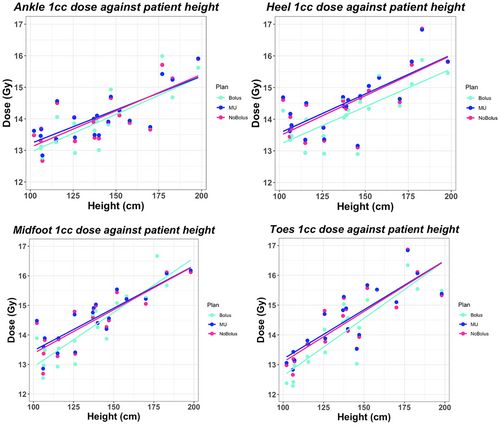
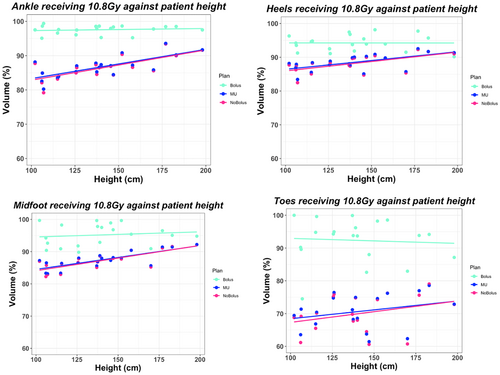
The median height and weight for each age group, under 5 years (A), 5–12 years (B), and over 12 years (C), was 106.15 cm and 18.0 kg (A), 137.25 cm and 28.05 kg (B), and 173.50 cm and 62.4 kg (C). Using Spearman's test across all regions of the foot, statistically significant rho coefficients (see Table 3) were produced for D1cc dose against patient height, weight and age, independent of bolus use (P < 0.05). This was reflected in the scatter plots presented in Figure 3 demonstrating a strong positive relationship between patient height and maximum dose. On average, patient height against D1cc, V90, and V110 dose metrics obtained the largest Spearman's coefficients (Table 3). Evaluating V90 coverage, a stronger correlation was depicted between patient height and coverage in the no bolus plans (rho = 0.57, P < 0.05) compared to the weak correlation depicted with bolus (rho = 0.02, P < 0.05), across all regions. This trend was observed in the scatter plots presented in Figure 4, by the reduced gradient of the fitted line for the bolus plan. In the toe region, for all plan types, a statistically insignificant correlation was evident (rho = 0.16, P = 0.22) for patient height against V90 coverage.
| Dose metric | Plan | Patient factor | Rho coefficient (Average across all regions) | P-value range (range across analysis of all regions; P < 0.05 is statistically significant) | |
|---|---|---|---|---|---|
| Min | Max | ||||
| D1cc | Bolus | Height | 0.80 | <0.001 | 0.004 |
| Weight | 0.76 | <0.001 | 0.002 | ||
| Age | 0.74 | <0.001 | 0.002 | ||
| No bolus | Height | 0.75 | <0.001 | 0.002 | |
| Weight | 0.73 | <0.001 | 0.004 | ||
| Age | 0.69 | <0.001 | 0.006 | ||
| MU | Height | 0.76 | <0.001 | 0.001 | |
| Weight | 0.73 | <0.001 | 0.004 | ||
| Age | 0.68 | <0.001 | 0.005 | ||
| V90 | Bolus | Height | 0.02 | 0.409 | 0.875 |
| Weight | 0.06 | 0.446 | 0.823 | ||
| Age | 0.04 | 0.502 | 1.000 | ||
| No bolus | Height | 0.57 | <0.001 | 0.121 | |
| Weight | 0.60 | <0.001 | 0.083 | ||
| Age | 0.56 | <0.001 | 0.123 | ||
| MU | Height | 0.59 | <0.001 | 0.118 | |
| Weight | 0.60 | <0.001 | 0.078 | ||
| Age | 0.55 | <0.001 | 0.136 | ||
| V110 | Bolus | Height | 0.62 | <0.001 | 0.154 |
| Weight | 0.58 | <0.001 | 0.245 | ||
| Age | 0.58 | <0.001 | 0.232 | ||
| No Bolus | Height | 0.57 | 0.001 | 0.057 | |
| Weight | 0.55 | <0.001 | 0.107 | ||
| Age | 0.53 | <0.001 | 0.132 | ||
| MU | Height | 0.55 | 0.004 | 0.081 | |
| Weight | 0.53 | 0.003 | 0.119 | ||
| Age | 0.50 | 0.001 | 0.155 | ||
- Abbreviations: rho, Spearman's correlation coefficient; D1cc, Minimum dose to the maximally irradiated 1.0 cm3; V90, relative volume (%) receiving at least 90% of the reference dose; V110, relative volume (%) receiving at least 110% of the reference dose; Bolus, original plan utilising ankle and foot bolus; No Bolus, retrospective plan with removal of bolus; MU, Monitor Unit; MU, retrospective plan with removal of bolus and adjustment of monitor units in an attempt to achieve similar dosimetry to ‘Bolus’ plan.
Discussion
Initial implementation of ankle and foot bolus with the MATBI technique at ROPART was based on radiation therapy principles as there is no reported data on its use in the treatment of TBI. This study aimed to determine if ankle and foot bolus was required to improve dose homogeneity of paediatric MATBI treatment by reducing hot spots within the ankles and heels while ensuring the toes received a minimum of 90% of the prescribed dose within the patient treatment plan.
Initial evaluation of dose metrics of each subregion of the foot and ankle demonstrated clinically insignificant variation in minimum dose (D1cc) received by maximally irradiated 1.0 cm3 regardless of plan type with minimal variation in median dose across all subregions. The greatest change in D1cc dose was observed in the heels, closely followed by the midfoot and the toes. The least variation was in the ankles, with less than a 0.1% increase in median dose when the bolus was removed. This interpretation indicates that bolus has minimal impact in reducing the maximum dose received in the feet and ankles, specifically the ankles, contrary to initial intent.
Dosimetric criteria utilised for uniformity evaluation in this study used the international standard TBI dose parameters of ±10% of the reference dose (V90 and V110).4 Assessment of the V85 and V115 (±15%) dose metrics were also considered to determine the impact of ankle and foot bolus on unfavourable dosimetry. The box plots and descriptive statistics demonstrated a greater change in the V85 and V90 coverage of each subregion, compared to the V110 and V115 coverage when the bolus was removed, indicating that removal of bolus had a greater effect on the percentage of the ankles and feet receiving the minimum required dose. Trends observed with the V85–V115 percentage volumes were consistent with those seen for the D1cc assessment. Specifically, removal of bolus had the largest effect on the toe region, followed by the ankles, midfoot, and heels. This could be due to the absence of tissue in the toe region, reducing the build-up effect and backscatter characteristics of the photon beam to achieve the desired coverage. There is great complexity in the modelling of the skin dose contribution in areas such as the ankles and feet, considering the angle of the foot and skin surface under the spoiler. Therefore, for the purpose of this study, the subsequent direct impact of the spoiler on dosimetry in the ankles and feet was not directly measured. Acknowledging the variation in contour and increased source-to-skin distances, the impact of the spoiler may have a clinical impact on the skin dose, however, the build-up region is needed to reach the maximum dose at depth and minimal required coverage. Therefore, the addition of bolus is suggested to provide the required build-up region in the ankles and toes, to achieve a minimum of 90% of the reference dose.
In attempting to meet dosimetric criteria without the use of bolus, optimisation of MU in a selection of beams traversing the ankle and foot region was performed. Standardly, ICRU recommends achieving 95–107% of the reference dose for optimal radiation therapy distribution.9 Acknowledging the specialised technique utilised in TBI treatment, as the reference dose is low and the PTV volume is large, achieving ±10% and higher hotspot doses are accepted. Maximum doses can range between 120% and 140%. These dose requirements were used when conducting the MU optimising process. Superior V100 coverage was achieved without bolus, and comparable V110 and D1cc were also achieved, however, the largest discrepancy in dose was depicted in the V90 coverage. This suggests that whilst equal dosimetry could be obtained in the no bolus plan for V100 and V110, MU adjustment alone was unable to produce comparable V90 dose coverage. Termination of MU adjustment iterations was decided when hotspots began to visually increase significantly in size to improve minimum dose coverage and reduce homogeneity. In instances where the beams traversed the entire ankle and foot region, there was limited optimisation opportunity to manipulate cold and hot doses in different regions separately. As such there is limited optimisation opportunity to manipulate hot and cold regions of the foot when the field includes the entire foot, and significant area superior to the foot. For this reason, future investigation into the modulation of parameters such as gantry angles and field sizes could be considered.
For all regions of the foot, height, weight, and age showed similar trends when compared to the various dose metrics, suggesting that all factors are closely related, as patients get older, they tend to grow taller and larger.
Scatter plots and Spearman's test indicated there was a weaker correlation between patient height and V90 coverage for the Bolus plan and a strong correlation with the D1cc dose. This suggests that the use of bolus does not influence the correlation between patient height and maximum dose, however, does remove the dependency of patient height to achieve V90 coverage. In the No Bolus plans, a stronger correlation between V90 coverage and patient height was present. This suggests that when bolus is not utilised, superior coverage in older patients can be achieved compared to the coverage obtained in younger patients. In the toe region for all plan types, a statistically insignificant correlation was depicted for height, weight, and age against V90 toe coverage (P-value > 0.05). As demonstrated in the scatter plots, the Bolus plan coverage improved by ~20% compared to the No Bolus and MU plans. This suggests that the extended distance from the source to the patient's toes, exacerbated in taller patients, has less impact on toe coverage, and instead is impacted greater by lack of tissue in the toe region, regardless of patient size. Similarly, results for patient weight showed visually similar findings to height. It is worthy to recognise the limiting nature of evaluating the relative volumetric data when comparing the difference in dose between areas of the foot. Given the physical difference in size standardly observed between the dimensions of the toes compared to the ankles, a reduction in the same cubic measurement of dose would represent a larger relative reduction in the toes compared to the ankles. By using the percentage of the volume receiving dose, variations in size within the same region are normalised due to differing ages.
Spearman's correlation test suggested height to have the strongest correlation to dose supported by visually stronger gradients in the scatter plots and slightly larger Spearman rho coefficients compared to weight and age for D1cc and V110. Future investigation into the effect of height is suggested, to further explore the extent of height as an explanation for dose. For example, bolus requirements may differ depending on age group. For example, smaller separation and circumference of foot size in younger patients may require bolus placement to be modified as opposed to a taller patient, resulting from cross sections of the treatment beams traversing at a larger extended SSD.
The sample size of 20 patients in this study is a limiting factor. It was deemed that due to the sample size and repeated measures, a P-value resulting from further statistical testing would be underpowered. As this is not a common procedure, treating only 3–10 patients per year, to obtain a larger sample size to perform additional statistical testing would require many more years' worth of patients. Patient selection was performed to achieve diverse height, weight, and age data for exploratory analysis, however, information addressing circumference of the ankle and patient separation specifically would have been useful to quantify patient predictors so dose differences against patient height could be better contextualised and reported. Unlike height and weight, this information was not recorded as part of routine clinical practice. Further, acknowledging the retrospective planning nature of this study, future work into dosimetric validation of results using phantoms or in-vivo dosimetry is recommended to support the TPS dose calculations. Another limitation of this study is the variability of bolus placement between clinical plans. Whilst all patients included in this study completed treatment with the utilisation of 1 cm of bolus, variations in bolus positioning, such as junctions between bolus pieces of the feet and ankles were observed and may have impacted heel and midfoot dosimetry. While there is a specified procedure for bolus construction, there is some variability between patients due to differing foot sizes with different foot flex, in an attempt to get the bolus to stay in place (Fig. 1). Improving coverage to achieve ±10% of the reference dose in TBI treatment is admirable for promoting good practice. This planning study has demonstrated that with reasonable intervention, through the use of bolus, admirable dosimetry is achievable without significant change to the organ at risk doses. The departmental protocol has been reviewed and will continue to be updated with the aim of greater consistency and standardisation, though it is acknowledged that some variations will occur due to patient-specific factors.
Conclusion
This study found that the current utilisation of ankle and foot bolus is necessary to achieve the primary dosimetric goal of a minimum dose of 90% of the prescribed dose (V90) to all subregions of the feet and ankles for all paediatric patients treated with MATBI. However, the initial rationale to utilise bolus for maximum dose reduction in the ankle region is not supported. When the bolus was removed comparable V100, V110, and D1cc dosimetry was possible however adequate V90 coverage was not possible without bolus even with MU optimisation, specifically in the toes. This study found a stronger correlation between height and maximum dose compared to weight and age in the ankles and feet regardless of bolus use. Future work assessing patient parameters such as ankle circumference and separation may be required to provide age thresholds and potential height indicators to determine patient-specific use.
Conflict of Interest
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




