Clinical validation of a semi-automated segmentation algorithm for target volume definition on planning CT and CBCT in stereotactic body radiotherapy (SBRT) for peripheral lung lesions
Abstract
Introduction
Stereotactic body radiotherapy (SBRT) is an ablative method for lung malignancies. Here, the definition of the gross target volume (GTV) is subject to interobserver variation. In this study, we aimed to evaluate the interobserver variability during SBRT and its dosimetric impact, as well as to introduce a semi-automated delineation tool for both planning computer tomography (P-CT) and cone beam CT (CBCT) to help to standardise GTV delineation and adaptive volume-change registration.
Methods
The interobserver variation of GTV manual contours from five physicians was analysed in 15 patients after lung SBRT on free breathing (FB) P-CT (n = 15) and CBCT (n = 90) before and after each fraction. The dosimetric impact from interobserver variations of GTV based on the original treatment plan was analysed. Next, the accuracy of an in-house easy-to-use semi-automated-segmentation algorithm for pulmonary lesions was compared with gold standard contours in FB P-CT and CBCT, as well as 4D P-CT of additional 10 patients.
Results
The interobserver variability in manual contours resulted in violations of dose coverage of the planning target volume (PTV), which, in turn, resulted in compromised tumour control probability in contours from four physicians. The validation of the semi-automated delineation algorithm using thorax phantom led to a highly reliable accuracy in defining GTVs. Comparing the unsupervised auto-contours with the gold standard delineation revealed high equal high concordance for FB P-CT, 4D P-CT and CBCT, with a DSC of 0.83, 0.76 and 0.8, respectively. The supervised use of the semi-automated delineation tool improved its accuracy, with DSCs of 0.86, 0.86 and 0.8 for FB P-CT, 4D P-CT and CBCT, respectively. The use of the algorithm was associated with a significantly shorter working time. The semi-automated delineation tool can accurately register volume changes in CBCTs.
Conclusion
The segmentation algorithm provides a reliable, standardised and time-saving alternative for manual delineation in lung SBRT in P-CT and CBCT.
Introduction
The role of ablative stereotactic body radiotherapy (SBRT) in early primary lung cancer and pulmonary metastases has evolved rapidly in the last few years. SBRT provides high local control rates comparable to surgical resection while being non-invasive.1-3 The development of SBRT was based on several advancement milestones in medical imaging and radiation treatment planning. Image-based planning and image-guided treatment delivery to detect and correct tumour motion allowed an improved therapeutic window through the highly conformal spatial dose distribution with an ablative dose to the target and rapid dose fall-off outside the target to minimise the radiation dose to the adjacent tissues. However, it is challenging in SBRT to develop a standardised and objective method for precise target volume definition, which will impede unnecessary volume expansion of the planning target volume (PTV) and a higher risk of geometric miss.4 The manual delineation could be time-consuming and highly prone to subjective differences in target delineation.
Multiple solutions for a standardised and more accurate volume delineation have been proposed: adding different imaging modalities, using standard contouring protocols, and the use of an automated contouring process were introduced.5 Several automated segmentation methods have been described, such as using voxel changes in identifying the edge or graph cuts, atlas-based automatic segmentation, and most recently, machine learning.6
At the same time, the application of cone beam computer tomography (CBCT) in radiation therapy enabled image guidance and, therefore, led to a marked improvement in high-precision radiotherapy. Although CBCT images provide the best real-time body information before irradiation, they are adversely affected by noise and artefacts, which degrade the image quality.7 In some circumstances, the image quality is so low that CBCT images cannot be used directly to re-evaluate the delineation of the tumour lesions and replan the dose distribution in adaptive radiotherapy.
This study aimed to validate the use of an easily applicable delineation algorithm for planning CT (P-CT) and CBCT by comparing the interobserver variability from manual and automated contouring of the gross target volume (GTV). Performing a pre-fractional and post-fractional CBCT, the intrafraction and interfraction variation of the GTV volume could be traced. Therefore, we investigated the potential role of automated delineation in standardisation and adaptive target volume delineation.
Material and Methods
Data acquisition
Free breathing (FB) P-CT and CBCTs from 15 patients with a total of 18 peripheral pulmonary lesions (three patients had two lesions) and four-dimensional (4D) P-CT from 10 patients with 10 pulmonary lesions who received SBRT for lung cancer or pulmonary metastases in the department of radiation oncology, RWTH Aachen university medical centre, were included in the current study (Table 1). Patients were selected according to the prescribed dose and the location of the lesion in the lung, being peripheral and not directly adjacent to surrounding structures like ribs or mediastinum. All lesions were analysed independently from each other. All patients were treated with a total dose of 45 Gy in three fractions of 15 Gy prescribed to the 67% isodose on alternative days, using a volumetric modulated arc therapy after signing informed consent for the treatment. Patients were immobilised using evacuated bags and transparent plastic films (BodyFIX 14 BlueBAG; BodyFIX Cover Sheet, ITV, Innsbruck, Austria). The breathing motion was limited passively using an abdominal press (BodyFIX Diaphragm Control ITV, Innsbruck, Austria). The initial free breathing 3-dimensional P-CT in 15 patients was performed on a 16-slice CT scanner (Brilliance CT Big Bore Oncology, Philips Medical Systems Inc., Cleveland, Ohio, USA) using 120 kV, 146 mAs, a pitch of 0.813 and a slice sickness of 3 mm (reconstructed pixel size 0.98 mm × 0.98 mm). During the simulation, the tumour motion was assessed using kV-fluoroscopy to ensure a motion-range ≤5 mm. Before and just after each treatment fraction, 3D CBCT images (XVI, Elekta, Stockholm, Sweden) were acquired using thorax imaging optimised conditions (120 kV and 264 mAs), resulting in a total of 90 CBCTs. The reconstructed pixel size was 1 × 1 mm with a slice thickness of 3 mm. The reconstructed CBCT images were automatically co-registered with the planning CT images using the bone registration algorithm within a clip-box. In a second step, manual registration was employed by aligning the lung lesion visualised in the CBCT centrally to the PTV from reference planning CT.
| Patient | Diagnosis | Histopathology | Staging | Site | No. of lesions | size of lesion-lesions cm3 |
|---|---|---|---|---|---|---|
| 1 (FB P-CT) | Lung cancer | Sq. cell Ca. | cT1 cN0 cM0 | Upper lobe, right | 1 | 1.726 |
| 2 (FB P-CT) | Lung cancer | NA (PET avid) | cT3 cN0 cM0 | Upper lobe, left | 2 | 18–6.5 |
| 3 FB P-CT | Lung cancer | NA (PET avid) | Middle lobe, right | 1 | 6.2 | |
| 4 FB P-CT | Metastasis | Oropharynx, Sq. cell. Ca. | IV | Upper lobe, left | 1 | 11.42 |
| 5 FB P-CT | lung cancer | NA (PET avid) | cT2 cN0 cM0 | Lower lobe, right | 1 | 1.38 |
| 6 FB-CT | Metastasis | Oropharynx, Sq cell. Ca | IV | Upper lobe, right | 2 | 3.4–1.4 |
| 7 (FB P-CT) | lung cancer | Adenocarcinoma | cT1 cN0 cM0 | Lower lobe, left- pleural contact | 1 | 3 |
| 8 (FB P-CT) | Lung cancer | Adenocarcinoma | cT1 cN0 cM0 | Upper lobe, right | 1 | 2.56 |
| 9 (FB P-CT) | Metastasis | Melanoma | IV | Lower lobe, left | 1 | 2.09 |
| 10 (FB P-CT) | Lung cancer | Adenocarcinoma | cT2 cN0 cM0 | Lower lobe, left | 1 | 20.49 |
| 11 (FB P-CT) | Lung cancer | NA (PET avid) | cT1 cN0 cM0 | Middle lobe, right | 1 | 3.28 |
| 12 (FB P-CT) | Lung cancer | Sq. cell Ca. | cT1 cN0 cM0 | Upper lobe, left | 1 | 2.471 |
| 13 (FB P-CT) | Lung cancer | Adenocarcinoma | cT2 cN0 cM0 | Upper lobe, right | 2 | 15–1.97 |
| 14 (FB P-CT) | Lung cancer | Adenocarcinoma | cT3 cN0 cM0 | Middle lobe, right | 1 | 3.83 |
| 15 (FB P-CT) | Metastasis | Melanome | IV | Middle lobe, right | 1 | 3.21 |
| 16 (4D P-CT) | Lung cancer | Adenocarcinoma | rcT1 cN0 cM0 | Upper lobe, right | 1 | 1.65 |
| 17 (4D P-CT) | Lung cancer | Adenocarcinoma | cT2 cN0 cM0 | Upper lobe, right | 1 | 13.76 |
| 18 (4D P-CT) | Lung cancer | Adenocarcinoma | cT2 cN2 cM0 | Middle lobe, right | 1 | 13.1 |
| 19 (4D P-CT) | Lung cancer | NA (PET avid) | cT1 cN0 cM0 | Upper lobe, left | 1 | 3.65 |
| 20 (4D P-CT) | Lung cancer | NA (PET avid) | cT1 cN0 cM0 | Upper lobe, left | 1 | 2.6 |
| 21 (4D P-CT) | Metastasis | Rectal cancer, Adenocarcinoma | IV | Middle lobe, right | 1 | 3.46 |
| 22(4D P-CT)) | Lung cancer | Adenocarcinoma | cT1 cN0 cM0 | Upper lobe, right | 1 | 13.76 |
| 23 (4D P-CT)) | Metastasis | Endometrial cancer, Adenocarcinoma | IV | Lower lobe, right | 1 | 2.97 |
| 24 (4D P-CT) | Metastasis | Oropharynx, Sq. cell. Ca. | IV | Upper lobe, right | 1 | 1.5 |
| 25 (4D P-CT) | Metastasis | Skin, Sq. cell. Ca. | IV | Lower lobe, left | 1 | 2.36 |
All CBCTs were sent back to the treatment planning system (TPS) Pinnacle3 (V.14.0; Philips Healthcare, Amsterdam, Netherlands), as the CBCTs were reconstructed in the P-CT image coordinates with corrections applied prior to export from the treatment machine, the CBCT images were correctly registered with the P-CT on import to replicate the delivered treatment field position.
For 4D P-CT, the reconstructed CT slices were binned into 10 phases. The Sentinel 4DCT (CRAD, Uppsala, Sweden) was used for motion tracking during the acquisition of the 4D P-CT. The maximum intensity projection CT (MIP) was used to delineate the internal target volume (ITV). FB P-CT, 4D P-CT and CBCT were anonymized for the purpose of the analysis. The current analysis was carried out in compliance with the regulation of the local ethical committee (Faculty of Medicine, RWTH Aachen University), and separate ethical approval was not required for the study.
The semi-automated delineation algorithm
An easy-to-use algorithm was implemented in the treatment planning system Pinnacle3 using the internal automated threshold delineation function and the region growing tool. The function requires the definition of a lower auto-contour threshold value in Hounsfield units (HU) to separate tumour from healthy tissue. The developed algorithm calculates this lower threshold automatically and can be applied to CT images and CBCT images without any change of parameters.
The algorithm was validated by comparing the known volumes from two tumour models in a thorax phantom (Dynamic Thorax Phantom, CIRS, Norfolk, USA) with the volumes defined by the semi-automated delineation algorithm for both imaging modalities; CT and CBCT. The phantom consists of a simplified human thorax geometry constructed of tissue and lung equivalent epoxy materials. The tumour models are water-filled spheres mounted on a rod, both consisting of Plastic Water® DT. The real tumour volumes were 10.12 and 2.90 cm3. The volumes delineated with the algorithm were: 9.94 ± 0.18 cm3 and 2.71 ± 0.07 cm3 for CT images 10.64 ± 0.32 cm3 and 2.97 ± 0.13 cm3 for CBCT images. The variation in volumes corresponded to a radius of <0.4 mm, which is below the pixel size within the data sets. The semi-automatic delineation of both tumour models was repeated 10 times to investigate the influence of manually defining the size of the bounding box. Accordingly, the variation of the delineated volume has a reproducibility of 4%, which corresponds to a variation in the radius of 1.4% (Tables S1 and S2).
Contour generation
Manual delineation of GTVs for all 18 lesions was generated within all data sets from FB P-CTs and CBCTs and ITV of 10 lesions using only the data sets from 4D P-CT (generated using MIP CTs) by an expert with experience of more than 20 years. These delineations were considered ‘gold standard labels’ for further evaluation. Next, five radiation oncologists with an experience range of 3–11 years manually delineated the GTV in each FB P-CT and CBCT image data set by adopting the delineation protocol from RTOG 08138 to reduce the interobserver variability and being blinded from the other contours. The delineation was performed using a standardised lung window level (window width 1601 HU, window level −300 HU). A mediastinal window (window width 401 HU, window level 800 HU) was also able to be used to ensure the exclusion of adjacent benign structures (vessel or chest wall) or atelectasis, these contours were named ‘manual contours’.
The unsupervised contours using the semi-automated delineation tool were named ‘unsupervised auto-contours’ and were generated by K.R and L.S.
Three months later, two radiation oncologists then evaluated the exact copy of the unsupervised semi-auto-contours. The radiation oncologists were allowed during the evaluation process to edit these contours to exclude any nearby vessels or bronchi. These contours were named ‘supervised auto-contours’.
Quantitative evaluation for manual contours and auto-delineation
The manual contours (FB P-CTs, CBCTs and 4D P-CT) from five physicians as well as the unsupervised and supervised auto-contours were evaluated for interobserver variability by assessing the spatial overlap to the gold standard dataset using the following indices:
The time needed for the delineation: The time necessary for the delineation of GTVs was recorded for all the physicians and the operator to run the auto-delineation algorithm for further evaluation.
Dosimetric and radiobiological treatment plan evaluation
Finally, the auto-contours volume and the dose covering 100% of GTV (D100-GTV) were calculated for all CBCT contours to evaluate the interfraction and intrafraction tumour volume and dose variation.
Statistical analysis
Correlation analysis with Spearman Rank Correlation was calculated using Free Statistics Software (v1.2.1).13 A one-sided paired T-test was used to evaluate the variation between D95-PTV, TCP-PTV, median CoV among the three fractions in CBCTs, and the time needed for contouring. Graphs were generated using Excel (Microsoft, CA, USA) and SPSS version 25 (IBM, New York, NY, USA).
Results
Interobserver variability in GTV delineation from P-CT and CBCTs
After the manual delineation of GTVs in FB P-CT, the mean and standard deviation of the CoV from the five physicians among the eighteen lesions was 0.17 ± 0.06 (Fig. S2a). Four patients (number 5, 7, 9 and 12) showed a higher CoV (>0.2), indicating a higher interobserver variation. Despite frequent higher CoV in smaller lesions, the statistical analysis revealed no correlation between the size of the lesions and the CoV (rho: −0.261 and 2-sided P-value: 0.347) (Fig. S2b). The mean CoV and standard deviation for manual delineations of lesions in CBCTs was 0.21 ± 0.1 (Fraction ‘Fr’ 1: 0.17 ± 0.08, Fr2: 0.21 ± 0.1, Fr3: 0.24 ± 0.11). The CoV for manual delineation of the GTVs in CBCTs was significantly increased in the second and third radiation fraction, compared to the first fraction, with p-value 0.005 and 0.002, respectively (Fig. S2c,d), indicating a poorer interobserver agreement during the course of treatment.
The mean DSC, which demonstrates the variation in the spatial overlap between contours from all five physicians and the ground truth contours in FB P-CTs for the 18 lesions, was 0.83 (Fig. S3a). The analogous mean DSC after manual contouring in CBCTs was 0.80 (Fig. S3b). The mean DSC for P-CT and CBCT will be used later to validate the auto-contours (unsupervised and supervised) and will be named ‘reference manual’.
Impact of interobserver variability on PTV size, D95-PTV and TCP-PTV
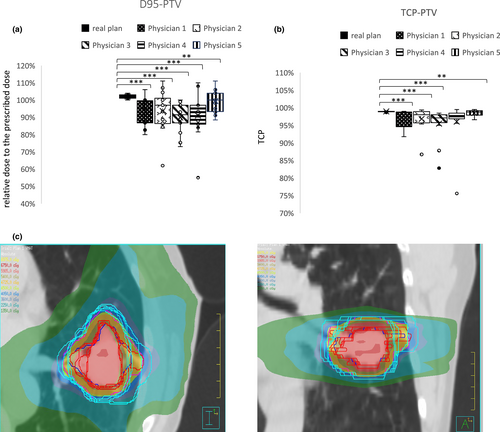
The resulting PTVs from manual GTV re-delineation in FB P-CTs were used to evaluate the corresponding dose-volume load based on the implementation of the new target volume delineation upon the original dose distribution of the initial radiation treatment plan for each patient (PTV-irradiated). Overall, the variation in manual delineations of the GTV among the 5 physicians led to variations in PTV size with an increase in mean PTV size from manual delineations in 12 patients, a decrease in two patients and a similar in one patient compared to PTV-irradiated (Table 2, Fig. 1c). These variations were translated into significantly lower D95-PTV than the original radiation plan for each patient for all manual contours (Fig. 1a). Consequently, these lower D95-PTV resulted in substantially lower TCP values for the re-planned dose application (Fig. 1b).
| Pt1 | Pt2 | Pt3 | Pt4 | Pt5 | Pt6 | Pt7 | Pt8 | Pt9 | Pt10 | Pt11 | Pt12 | Pt13 | Pt14 | Pt15 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PTV Physician 1 | 13.61 | 105.2 | 37.26 | 31.44 | 13.34 | 38.9 | 24.38 | 22.7 | 15.26 | 91.3 | 20.87 | 18.9 | 88.9 | 25 | 21 |
| PTV Physician 2 | 12.9 | 118 | 31.52 | 30.35 | 15.36 | 37 | 20.75 | 24.18 | 18.9 | 97.6 | 20.6 | 16.64 | 66.57 | 23.81 | 21.3 |
| PTV Physician 3 | 12.75 | 130.4 | 34.83 | 29.55 | 16.37 | 42.7 | 31.62 | 26.84 | 16.41 | 79.42 | 21.32 | 17.56 | 97.08 | 26.6 | 24.2 |
| PTV Physician 4 | 13.24 | 108.31 | 30.11 | 27.15 | 14.45 | 43.5 | 22.9 | 24.01 | 22.94 | 79.94 | 24.36 | 24.12 | 91.3 | 28.89 | 22.5 |
| PTV Physician 5 | 13.35 | 94.83 | 31.33 | 22.06 | 10.96 | 32.63 | 19.8 | 19.9 | 13.9 | 65.35 | 20.86 | 16.92 | 71 | 23.23 | 20.3 |
| Mean (SD) | 13.17 (±0.35) | 111.35 (±13.48) | 33.01 (±2.95) | 28.11 (±2.08) | 14.1 (±2.08) | 38.95 (±4.43) | 23.89 (±4.68) | 23.57 (±2.54) | 17.48 (±3.56) | 82.77 (±12.40) | 21.6 (±1.56) | 18.83 (±3.08) | 82.97 (±13.38) | 25.51 (±2.29) | 21.86 (±1.53) |
| PTV-irradiated | 10.11 | 80.57 | 39.47 | 20.79 | 12.45 | 27.46 | 29.95 | 23.33 | 12.477 | 70.6 | 18.1 | 17.45 | 50.64 | 18.48 | 20.3 |
The accuracy of the semi-automated delineation tool
The median DSC for the unsupervised auto-contours in FB P-CT was 0.83, and the median of DSC for the supervised auto-contours was 0.86 (Fig. 2a). Further, the median of DSC for unsupervised auto-contours and supervised auto-contours in 4D P-CTs were 0.76 and 0.86, respectively (Fig. 2a). Also, the median of DSC for the unsupervised auto-contours and supervised auto-contours in CBCTs were 0.80 (Fig. 2b), indicating an acceptable accuracy of the delineation algorithm in both P-CT and CBCT.
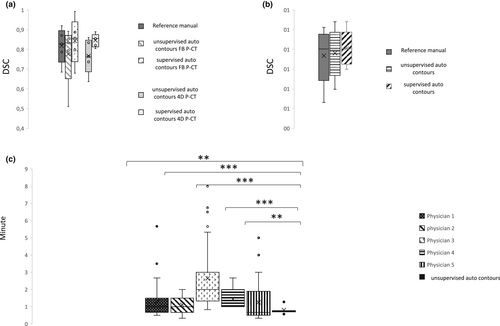
Furthermore, the time consumed for the algorithm's delineation was significantly and stably lower (0.81 ± 0.2 min) than all five physicians' time (1.3 ± 1, 1.1 ± 0.52, 2.66 ± 1.89, 1.46 ± 0.5 and 1.27 ± 1.12 min) (Fig. 2c).
The tracking of the interfraction and intrafraction GTV volume changes
Based on the pre-radiation (pre-RT) images for each fraction, the evaluation of the interfraction GTV volume changes during radiotherapy, between fractions 1&2 and fractions 2&3, showed an increase in GTV volume in most patients. While a mean volume increase of 33.4% (range 6.3–72.1%) was observed in 11 patients, only four patients revealed a lesser pronounced decrease in tumour volume (4.6%, range 2.7–6.7%). The ratio of patients with volume increase and decrease was equal between unsupervised auto-and gold standard contouring; however, the mean value in volume changes was higher after semi-automatic segmentation (fraction 1–2 11.6% vs. 18.4%; fraction 2–3 18.4% vs. 23.25%) (Fig. 3a). The difference in GTV volume data between semi-automated segmentation and gold standard delineation was non-significantly different (the mean: 14.6% manual contouring, 20.5% automatic segmentation). The standard deviation was equal for both delineation methods (17.5% and 17.8%) with a broad range of percent volume changes.
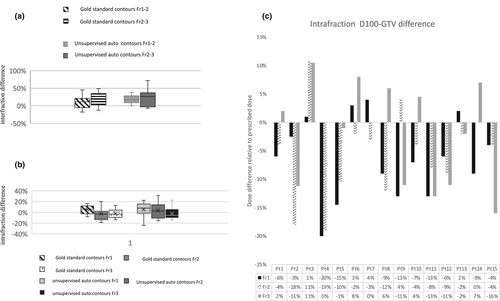
The intrafraction GTV volume changes were evaluated using the pre-RT and immediate post-RT CBCTs for the corresponding fraction during radiotherapy. Overall there was an intrafractional volume decrease both in the gold standard and unsupervised automatic delineation (Fig. 3b). semi-automated delineation revealed a decreased GTV volume immediately after SBRT in 4 patients for fraction 1, 5 patients for fraction 2 and 10 patients for fraction 3, and gold standard delineations in 4, 9 and 9 patients, respectively. The standard deviation demonstrated a broader range of values for both contours (7.85 and 14.14%).
Evaluating the impact of the intrafraction tumour volume changes (using the unsupervised auto-contours) on the initial treatment plan resulted in an underdosing of mean D100-GTV of 9.45% ±5.2% in 11 patients within the 1st fraction, 7.95% ±5.94% in 12 patients in the 2nd fraction and 9.31% ±5.63% in 7 patients in the 3rd fraction (Fig. 3c).
Discussion
In the current study, we emphasised the impact of the interobserver variation of GTV delineation in SBRT of pulmonary lesions through the evaluation of its effect on the dose coverage of the target volumes based on the original radiation plan for each patient. Unsurprisingly, we found that the interobserver variation in the GTV delineation (manual contours) in a cohort of 15 patients resulted in a systematic and significant underdosing for the PTVs from all the participating physicians, which significantly lower TCP at least in the contours from four physicians.
The mean CoV among all five observers in FB P-CTs was 0.17 ± 0.06. The interobserver variation was high in contours of patient number 5,7,9 and 12 in P-CTs with CoV > 0.2. While all those four lesions were relatively small (size ≤ 3cm3), the correlation between CoV and the size of the lesions was not statistically significant in contrast to what was previously reported by Altoraji et al.20 The target volume delineation in CBCTs was more challenging, with more frequent high CoV. In particular, we observed a statistically significant higher interobserver variation in the later CBCTs and fractions. We do not have an explanation for such an observation. Still, a possible suggestion may be the increased radiation-induced changes in the lung parenchyma or tumour deformation through the course of the irradiation, which reduces the sharpness of tumour boundaries.
With improvements in the conformal dose application, the impact of the interobserver variability in target volume delineation evolved into a significant issue in radiation oncology.14-16 The main concerns are missing part of the target volume or involving normal tissue in target volumes. Subsequently, this could result in undermining tumour control, as we emphasised in the current study or increasing toxicities.17, 18 Also, interobserver variability could impact the robustness of the radiomics features negatively, and the standardisation of target volume delineation could improve the stability of the prognostic value of the radiomics features.19
In the current study, we introduced a semi-automated delineation tool for target volume delineation based on the change of HU units to define the tumour boundaries using a function available in the TPS Pinnacle,3 which is easily applicable in the clinical environment within the TPS. This semi-automated delineation tool is a simple approach that uses the HUs in the lesion and the surrounding tissue to define the lower threshold, which makes it more patient-specific than other approaches using a fixed HU Threshold,21 therefore, can be used for P-CT datasets and CBCT datasets. The algorithm's accuracy was validated using a lung phantom with known lesion size. Using the algorithm, the variation in the radius of the delineated lesion size on imaging is <0.4 mm, which is smaller than the pixel size of the image datasets. The accuracy and repeatability are in the same order as other automated delineation algorithms.21, 22
For further validation of the semi-automated delineation algorithm, we examined the DSC among the five physicians as human operators in reference to ground truth labels in 15 FB P-CTs and 19 CBCTs. Further, we calculated the mean of all the medians DSCs from all five operators in both FB P-CT and CBCTs to be used as a ‘reference manual’ to validate the contours from the algorithm, which were 0.83 and 0.80, respectively. The median DSC for the auto-contours from the algorithm in FB P-CTs and CBCTs was 0.83 and 0.80, respectively, indicating that auto-contours are clinically reliable even in limited quality images as CBCTs.
We experienced some shortcomings in the delineation algorithm, for example the inclusion of an adjacent small vessel within the GTV (Fig. S4). To overcome such a problem, we allowed a modification of the auto-contours by the agreement of two observers 3 months after the manual delineation to ensure less influence of the personal judgement based on the previous contouring process. This improved the median DSCs in P-CT, enhanced the lower values and reduced the range in DSCs in CBCTs.
Further, we examined the semi-automated delineation tool using 4D P-CT. For this purpose, we included a second cohort of 10 4D P-CTs, and the delineation was carried out in MIP CTs. Here, the DSCs for the auto-contours and modified auto-contours were 0.76 and 0.86, respectively.
Nevertheless, we believe that applying an automated delineation method should be simple and does not consume time besides the accuracy to improve its feasibility in routine application. Indeed, we showed that the application of the current semi-automated delineation tool significantly reduced the time needed for the delineation process in a stable manner compared to the manual delineation adding to its value.
Another possible application of the semi-automated delineation tool is tracking the interfraction and intrafraction changes and deformations. The tumour interfraction, intrafraction deformation and size variation have been widely discussed in lung SBRT.21, 23 Theoretically, a reduction in the size would not negatively impact the dosimetric outcome. In contrast, the increase in the size of the irradiated lesion compared to the initial P-CT may adversely affect the dose distribution in PTV.24, 25 Another study retrospectively observed the interfraction tumour volume change in twenty-two patients. It concluded a correlation between a marked reduction in the size during the SBRT in early lung cancer and worse clinical outcomes, progression-free survival and overall survival.26 Repeated CBCT before and after each fraction has been proposed to address such a problem as a tool for the target volume monitoring in lung SBRT.27 The addition of an automated method to delineate the target volume would help to figure out the magnitude of intrafraction and interfraction tumour deformations and the necessity to repeat the planning in case of undesired dosimetric violations, known as adaptive radiation therapy (ART).28 In the current study, we tested the semi-automated delineation tool in detecting such variation over repeated CBCTs before and after each fraction and the dosimetric difference that resulted from such variation on D100-GTV.
Indeed, we observed a deformation over the multiple CBCTs with an increase in GTV volume in 60% of the patients, a reduction in the volume in the subsequent CBCTs in 13.4% of the patients and mixed variation in the size (increase and decrease) in 26.66% of the patients. We cannot explain with certainty these changes in the volume of GTVs in CBCTs, but a possible contribution to such changes could be the irregularity of breathing patterns during the radiation course.29 Still, the automated contouring for online and speed delineation of the GTV in CBCT would provide a tool for detecting intrafraction and interfraction volume changes that could be better compensated in the calculation process of PTV margins.
Limitations of the Study
There are some limitations of the current study that should be openly addressed. the semi-automated delineation tool can be applied mainly for the peripheral lesions without large contact with the chest wall or mediastinum, as it cannot accurately differentiate between the tumour boundaries and thorax wall or mediastinum. This would reduce the applicability of the tool. The necessity to revise the delineation due to the possible inclusion of nearby vessel is another limitation of the tool. Finally, the need to define ROI to apply the tool would make the tool semi-automated rather than a fully automated tool.
Conclusion
Taken together, the supervised use of the aforementioned semi-automated delineation tool provides a clinically reliable and standardised target volume delineation tool in both P-CT and CBCT in peripheral lung SBRT. It is user-friendly, saves time and can detect any deformation or volume changes of the targets over the radiation course, allowing the chance for adaptive consideration of the target volume.
Funding information
Open access funding enabled and organised by Projekt DEAL.
Conflict of interest
All authors declare that they have no competing interests.
Acknowledgement
Open Access funding enabled and organized by Projekt DEAL.
Open Research
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.




