Does the Internal Carotid Artery Attenuate Blood-Flow Pulsatility in Small Vessel Disease? A 7 T 4D-Flow MRI Study
Abstract
Background
Increased cerebral blood-flow pulsatility is associated with cerebral small vessel disease (cSVD). Reduced pulsatility attenuation over the internal carotid artery (ICA) could be a contributing factor to the development of cSVD and could be associated with intracranial ICA calcification (iICAC).
Purpose
To compare pulsatility, pulsatility attenuation, and distensibility along the ICA between patients with cSVD and controls and to assess the association between iICAC and pulsatility and distensibility.
Study Type
Retrospective, explorative cross-sectional study.
Subjects
A total of 17 patients with cSVD, manifested as lacunar infarcts or deep intracerebral hemorrhage, and 17 age- and sex-matched controls.
Field Strength/Sequence
Three-dimensional (3D) T1-weighted gradient echo imaging and 4D phase-contrast (PC) MRI with a 3D time-resolved velocity encoded gradient echo sequence at 7 T.
Assessment
Blood-flow velocity pulsatility index (vPI) and arterial distensibility were calculated for seven ICA segments (C1–C7). iICAC presence and volume were determined from available brain CT scans (acquired as part of standard clinical care) in patients with cSVD.
Statistical Tests
Independent t-tests and linear mixed models. The threshold for statistically significance was P < 0.05 (two tailed).
Results
The cSVD group showed significantly higher ICA vPI and significantly lower distensibility compared to controls. Controls showed significant attenuation of vPI over the carotid siphon (−4.9% ± 3.6%). In contrast, patients with cSVD showed no attenuation, but a significant increase of vPI (+6.5% ± 3.1%). iICAC presence and volume correlated positively with vPI (r = 0.578) in patients with cSVD and negatively with distensibility (r = −0.386).
Conclusion
Decreased distensibility and reduced pulsatility attenuation are associated with increased iICAC and may contribute to cSVD. Confirmation in a larger prospective study is required.
Evidence Level
2
Technical Efficacy
Stage 2
Increased cerebral blood-flow pulsatility is associated with stroke risk, cognitive impairment, and cerebral small vessel disease (cSVD), including lacunar infarcts and deep intracerebral hemorrhage (dICH).1 Pulsatility is increased in patients with hypertension, which is an independent risk factor for stroke and cSVD.2-4 Maintaining normal cerebral flow pulsatility depends on effective attenuation of the cardiac pulse pressure along the arterial tree.5 This attenuation involves multiple components, including damping by the aortic wall; damping at distal branches6 and wave reflections due to different impedances in these branches.7 Recently, the internal carotid artery (ICA) siphon was identified as an attenuator for intracranial pulsatility,.8, 9
Insufficient pulsatility attenuation along the ICA may be a contributing factor to the development of cSVD. Intracranial ICA calcifications (iICAC) occur predominantly in the internal elastic lamina10 of the carotid siphon,11 yielding increased vessel wall stiffening and reduced pulsatility attenuation over the carotid siphon, which in turn is expected to contribute to increased microvascular pulsatility.12 Increased pulsatility in cerebral perforating arteries in patients with cSVD has been reported, using the high sensitivity of 7 T MRI,13 but the pulsatility and distensibility along the ICA in patients with cSVD, and the influence of iICAC, is largely unknown. 4D-flow measurements, enable us to analysis all ICA segments instead of preset 2D-flow locations and better has accessibility than ultrasound.9, 14
Thus, the aim of this explorative cross-sectional study was to investigate whether pulsatility attenuation over the ICA is reduced in cSVD, and if reduced attenuation is associated with iICAC. Additionally, we aimed to assess the influence of iICAC on pulsatility and distensibility in patients with cSVD.
Methods
Study Participants
The institutional review board approved the study and all subjects provided written informed consent. All participants gave informed consent for both the first study and using their available data for subsequent research. The current study is a post hoc analysis on a previous 7 T MRI study,13 which aimed at visualizing the perforating arteries in cSVD patients, for which 7 T MRI indisputably has added value over 3 T MRI.15 They evaluated pulsatility in penetrating intracranial arteries in 21 patients with cSVD with a lacunar infarction or a spontaneous dICH, attributed to cSVD according to clinical guidelines16 and 18 age- and sex-matched controls. The combination of patients with lacunar infarction and dICH into a single group representative for cSVD for the current study was justified by performing a subgroup analysis, which did not show differences in velocity pulsatility, mean velocity, and arterial distensibility between the subgroups (Supplementary Table S1). Controls with a history of neurological disease or with silent cSVD on MRI, defined as white matter hyperintensities (Fazekas scale ≥2), lacunar infarction or dICH, were excluded.
For the current study, we selected patients with cSVD and controls with complete 7 T 4D phase-contrast MRI (4D PC-MRI) datasets. None of the patients had a >50% ICA stenosis on CT angiography (CTA) or had undergone carotid artery stenting or endarterectomy. Blood pressure was measured just before the 7 T MRI (the last measurement out of three being used in analyses). Hypertension was defined as a blood pressure above 140/90 mm Hg and/or use of antihypertensive drugs. Hypercholesterolemia was defined as previously diagnosed or current statin use. Other cardiovascular disease recorded included history of myocardial infarction, peripheral arterial disease, aneurysm, cardiac arrhythmia, heart failure, or valvular heart disease. Smoking was recorded as the cumulated pack-years.
MRI Measurements
Participants underwent 7 T MRI (Philips Healthcare, Best, The Netherlands) using a volume transmit and 32-channel receive coil (Nova Medical, Houston, USA). The scan protocol included a 3D T1-weighted gradient echo scan with the following scan parameters: acquired resolution 1.0 × 1.0 × 1.0 mm3, TR/TE = 4.2/1.8, field of view (FOV) 300 (feet-head) × 190 (right–left) × 248 (anterior–posterior) mm3 and acquisition duration was 2 minutes. Second-order image-based shimming as available on the scanner was applied during all image acquisitions to mitigate B0-field inhomogeneity at 7 T. Only the 4D PC-MRI acquisition over the ICA trajectory was used for the current study.13 The 4D PC-MRI acquisition was planned on the 3D T1-weighted gradient echo and covered both ICAs, preferably including all seven segments from the extracranial C1 (below the skull base) to intracranial C7 (terminal segment). Time-resolved 3D phase-contrast velocity maps over the cardiac cycle were acquired for three orthogonal velocity encodings separately (feet–head, right–left, and anterior–posterior). Parameters for 4D PC-MRI were acquired resolution 0.8 × 0.8 × 0.8 mm3, velocity encoding sensitivity 100 cm/sec, angulated coronal FOV 250 (feet–head) × 190 (right–left) × 20 (anterior–posterior) mm3, flip angle 15°, and acquired temporal resolution 65 msec. Retrospective gating used a peripheral pulse unit for heartbeat detection. Acquisition duration was 4:55 min:sec for each direction of velocity encoding for a heart rate of 60 beats per minute.
Suitable 4D-flow datasets were available for 17 out of 21 patients with cSVD (7 with lacunar infarct; 10 with dICH) and 17 age- and sex-matched controls. Not all ICA segments were always included due to the small anterior–posterior FOV, giving an uneven number of carotid measurements. Patients with cSVD more often had hypertension and hypercholesterolemia (Table 1), but no difference in blood pressure at the time of the MRI scan (systolic, P = 0.39; diastolic, P = 0.35).
| cSVD (n = 17) | Controls (n = 17) | P value | |
|---|---|---|---|
| Men (%) | 14 (82) | 15 (88) | 0.63 |
| Age, years (SD) | 60 (10) | 62 (9) | 0.52 |
| BMI, kg/m2 (SD) | 26 (4.9) | 25 (2.1) | 0.51 |
| Hypertension (%) | 14 (82) | 5 (29) | <0.01 |
| Systolic/diastolic blood pressure (SD) | 136/84 (22/9) | 131/81 (14/9) | 0.39/0.35 |
| Heart rate (SD) | 70 (12) | 67 (15) | 0.49 |
| Hypercholesterolemia (%) | 11 (65) | 5 (29) | 0.04 |
| Other cardiovascular disease (%) | 4 (24) | 3 (18) | 0.67 |
| Pack-years smoking (median [IQR]) | 5 [0–26] | 0 [0–11] | 0.18 |
| WMH Fazekas scale (range) | 2 (1–3) | 1 (0–1) | - |
- BMI = body mass index; cSVD = cerebral small vessel disease; IQR = interquartile range; NA = not applicable; SD = standard deviation; WMH = white matter hyperintensities.
Image Processing
4D PC-MRI datasets were analyzed (by RvT with >3-years of experience with 4D flow analysis) using CAAS MR Solutions v5.1.1 software (Pie Medical Imaging, Maastricht, The Netherlands). Automatic background phase correction and antialiasing correction was performed using the CAAS software for all 4D PC-MRI datasets as described elsewhere.17 CAAS automatically generates the centerline and perpendicular slices for all seven ICA segments as defined by Bouthillier et al18 (Fig. 1). These perpendicular slices were visually checked and automatically propagated to create volumetric flow rate traces and separate velocity and area traces over the cardiac cycle. The blood-flow velocity pulsatility index (vPI = (Velocitymax − Velocitymin)/Velocitymean) was calculated from each velocity trace. Arterial distensibility = (ΔA/(A*ΔP)) in kilopascal (kPa)−1 was calculated from each area curve, where A indicates the area of the arterial lumen region of interest (ROI) at end-diastole, ΔA is the stroke change in lumen area over the cardiac cycle and ΔP is (systolic pressure – diastolic pressure).19 The diameter at end diastole was calculated from A, assuming a circular ROI. vPI, arterial distensibility, mean velocity, and diameter were calculated for all seven ICA segments (C1–C7).18
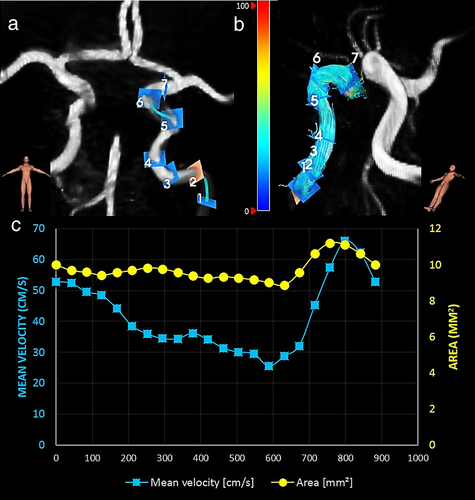
The interexamination, interobserver reproducibility, and intraobserver repeatability of the analysis with the CAAS software have been tested previously and showed a very good reproducibility and repeatability for volume, surface area, and centerline length (ICC = 0.65–0.96) and excellent reproducibility and repeatability for maximal diameter (ICC = 0.94–0.99).20-22 For the current study, we assessed the intraobserver repeatability (RvT with 3 years of experience with MRI flow data analysis, including CAAS software) by analyzing five subjects twice. The results showed an average dice coefficient of 0.94 (range 0.90–0.98) for vPI, mean velocity, diameter, and arterial distensibility.
CT Measurements in Patients With cSVD
Brain CT and neck CTA was standard clinical care for all included patients with cSVD but was not performed in controls as unnecessary radiation exposure was considered unethical. CT/CTA in patients with cSVD was used to determine carotid stenosis and presence and volume of iICAC from C3 to C7 (C1–C2 segments were outside the noncontrast CT coverage) by a radiologist with 23 years of experience (B.V.). The calcification volume was assessed using the Agatston method for coronary artery calcification on a workstation (IntelliSpace, Philips Healthcare).23 Interobserver reproducibility and intraobserver reproducibility for iICAC volume scoring were assessed by three independent observers, each of whom analyzed all available CT images of the included cSVD patients. Observer 1: B.V. with 23 years of experience scored all subjects twice, with an interobserver variability of 0.982 for scoring iICAC volume. An ICC of 0.994 was obtained between IvdS (second observer with 20 years of experience) and BV for scoring iICAC volume. The ICC was 0.983 between RvT (fourth year PhD student) and B.V. for scoring iICAC volume. This shows excellent intraobserver and interobserver reproducibility in scoring iICAC volume, which is in line with the literature.24 The first measurements of observer 1 (B.V.) were used for this analysis.
Statistical Analysis
Statistical analysis was performed in SPSS (Version 25, IBM, Armonk, NY, USA). Normality of data was tested using the Kolmogorov–Smirnov test of normality and differences in vPI and distensibility were assessed using independent samples t-tests between the patients with cSVD and controls for every ICA segment. To adjust for influence of age, sex, hypertension, and hyperlipidemia on both vPI and distensibility in both groups, a linear mixed-effect model with vPI and distensibility as mixed effects was used with separate measurements for both left and right ICAs. Since pulse pressure is an independent determinant of arterial distensibility,25 we included systolic and diastolic blood pressure instead of hypertension in a linear mixed-effect model to study the potential influence and/or relationship between blood pressure, vPI, and arterial distensibility.
Influence of presence and volume of calcification on vPI and distensibility was evaluated in the cSVD group with correction for age, sex, hypertension, and hyperlipidemia using a linear mixed-effect model. The relation between iICAC volume and vPI and distensibility was assessed for left and right ICA separately with Pearson's correlation coefficient (r), using the mean vPI and distensibility over C3–C7 for each ICA. The correlation between iICAC and vPI attenuation from C1 to C7 of the ICA was also studied. The threshold for statistical significance was P < 0.05 (two tailed).
Results
vPI and Distensibility Changes Along the ICA in Patients With cSVD and Controls
vPI and distensibility were similar between right and left ICA in both the patient and control groups (see Supplemental Table S2) and are therefore reported as one mean value per segment. The mean blood flow along the ICA was significantly lower in patients with cSVD (3.36 ± 0.43 mL/sec) compared to controls (3.61 ± 0.41 mL/sec).
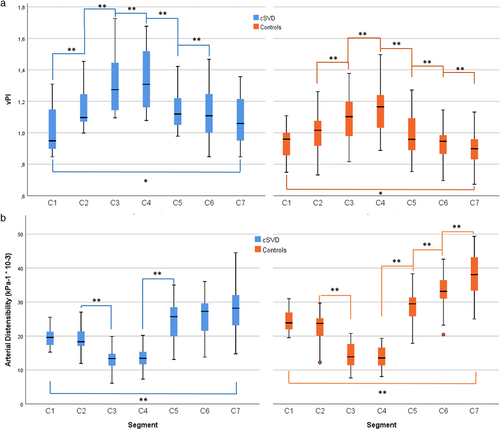
In both cSVD and control groups, vPI increased from the extracranial segment C1 over the bony carotid canal (C3) to C4 and then decreased over the carotid siphon toward the terminal intracranial segment C7 (Fig. 2 and Table 2). vPI was significantly higher in patients with cSVD compared to controls in segments C2–C7, but there was no significant difference in segment C1 (P = 0.102). Conversely, distensibility and mean velocity decreased from C1 to C4 and then increased toward C7. Distensibility was significantly lower in patients with cSVD for segments C1, C2, C5, C6, and C7 but not for C3 and C4 (close to the bony carotid canal) (P = 0.680 and P = 0.342, respectively). The lumen area pulsatility is provided in Supplementary Table S3 and showed similar differences between patients and controls and a similar trend along the ICA segments. Multilevel analysis showed that vPI and distensibility were directly associated (regression coefficient [standardized beta] for vPI vs. distensibility = −0.68 [−0.94; −0.43]), showing that by a single step increase of the determinant (distensibility), the vPI decreases with 0.68. This means that higher distensibility was accompanied by lower velocity pulsatility per segment. No statistically significant differences between groups were found for Vmean (Table 3). The arterial diameter was significantly higher in the control group for segments C2–C6, but this difference was not significant for C1 (P = 0.209) (Table 3).
| cSVD | Controls | P-value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ICA segment | Na | vPI | Change to C1 (%) | Distensibility (kPa−1*10−3) | N | vPI | Change to C1 (%) | Distensibility (kPa−1*10−3) | vPI | Distensibility |
| C1 | 16 | 1.02 ± 0.15 | 19.69 ± 2.85 | 14 | 0.94 ± 0.10 | 24.80 ± 3.64 | 0.102 | <0.001 | ||
| C2 | 24 | 1.16 ± 0.14 | +14.5 | 19.53 ± 3.81 | 25 | 1.00 ± 0.12 | +6.5 | 22.64 ± 4.59 | <0.001 | 0.013 |
| C3 | 21 | 1.32 ± 0.20 | +29.2 | 13.12 ± 3.35 | 28 | 1.09 ± 0.15 | +16.3 | 14.22 ± 3.71 | <0.001 | 0.290 |
| C4 | 29 | 1.35 ± 0.19 | +32.6 | 14.11 ± 3.66 | 31 | 1.16 ± 0.15 | +23.4 | 13.82 ± 3.07 | <0.001 | 0.740 |
| C5 | 25 | 1.15 ± 0.12 | +12.6 | 24.03 ± 6.45 | 25 | 0.99 ± 0.14 | +5.4 | 28.67 ± 4.86 | <0.001 | 0.006 |
| C6 | 31 | 1.12 ± 0.16 | +9.7 | 26.27 ± 6.50 | 31 | 0.93 ± 0.10 | 0.0 | 33.91 ± 5.39 | <0.001 | <0.001 |
| C7 | 33 | 1.09 ± 0.15 | +6.5 | 28.01 ± 7.14 | 32 | 0.89 ± 0.10 | −4.9 | 37.93 ± 6.58 | <0.001 | <0.001 |
- P values refer to segment comparison between cSVD and controls group.
- a Number of combined right and left carotids.
- kPa = kilo Pascal.
| cSVD | Controls | P value | ||||||
|---|---|---|---|---|---|---|---|---|
| ICA segment | Na | Vmean | Diameter | N | Vmean | Diameter | Vmean | Diameter |
| C1 | 16 | 21.7 ± 4.9 | 3.49 ± 0.36 | 14 | 22.7 ± 5.0 | 3.65 ± 0.25 | 0.428 | 0.209 |
| C2 | 24 | 23.2 ± 5.9 | 3.39 ± 0.39 | 25 | 19.6 ± 5.5 | 3.65 ± 0.36 | 0.663 | 0.038 |
| C3 | 21 | 21.2 ± 7.4 | 3.18 ± 0.41 | 28 | 19.2 ± 5.7 | 3.58 ± 0.42 | 0.911 | 0.001 |
| C4 | 29 | 18.2 ± 4.6 | 3.18 ± 0.37 | 31 | 18.5 ± 6.0 | 3.49 ± 0.41 | 0.620 | 0.003 |
| C5 | 25 | 25.7 ± 5.5 | 3.13 ± 0.29 | 25 | 24.6 ± 7.2 | 3.33 ± 0.40 | 0.852 | 0.048 |
| C6 | 31 | 30.5 ± 6.5 | 3.01 ± 0.37 | 31 | 31.9 ± 6.7 | 3.23 ± 0.43 | 0.385 | 0.033 |
| C7 | 33 | 34.3 ± 8.4 | 2.96 ± 0.30 | 32 | 38.9 ± 6.9 | 3.05 ± 0.36 | 0.363 | 0.209 |
- a Number of combined right and left carotids.
The control group showed a significant net attenuation in vPI over the ICA: (vPIC7-vPIC1 = −4.9% ± 3.6%; average of both ICAs). In contrast, patients with cSVD displayed a statistically significant increase in vPI over the ICA: (vPIC7-vPIC1 = +6.5% ± 3.1%). vPI was positively associated with hypertension in both groups. After correcting for age, sex, hypertension, and hyperlipidemia using the mixed-model analysis, the cSVD group still had a higher vPI: beta (95% CI) 0.18 (0.15; 0.21) and a lower distensibility: beta (95% CI) −5.7 × 10−3 kPa−1 (−4.4 × 10−3; −6.9 × 10−3) compared to controls. Also, after including the systolic and diastolic blood pressure as covariates, patients with cSVD had higher vPI: beta (95% CI) 0.19 (0.16; 0.22) and lower distensibility: beta (95% CI) −5.0 × 10−3 kPa−1 (−4.1 × 10−3; −5.7 × 10−3) than controls.
Calcification Effects on vPI and Distensibility in Patients With cSVD
One patient was excluded for calcification analysis due to inadequate CT. Carotid calcification was present in 10 of the 16 patients with cSVD (62.5%), correlating positively with vPI (r = 0.578) and negatively with distensibility (r = −0.386) in both ICAs. Calcification volume (mean 107 ± 95 mm3) correlated with vPI in the right (r = 0.645) and left ICA (r = 0.633) and with distensibility in both right (r = −0.411) and left ICA (r = −0.407). After correcting for covariates using the mixed model analysis, the relation between vPI and calcification (beta [95% CI] 1.27 [1.23; 1.31]) and between distensibility and calcification (beta [95% CI] −20.6 × 10−3 [−18.8 × 10−3; −22.5 × 10−3]) remained. The change in vPI between C1 and C7 (i.e. vPIC7-vPIC1) correlated negatively with calcification volume (r = −0.559) and with age (r = −0.211). Patients with cSVD without iICAC also had significantly higher vPI (mean vPI is 1.11 [mean whole cSVD group is 1.17]) than the controls (mean vPI 1.00).
Discussion
This study used 4D flow on 7 T MRI and demonstrated that patients with cSVD have higher vPI values and lower distensibility values along the ICA compared to controls. No effective vPI attenuation by the carotid siphon was seen in patients with cSVD but, rather, a statistically significant increase that was independent of age, sex, hypertension, and hyperlipidemia. vPI correlated positively, and distensibility negatively, with iICAC presence and volume in patients with cSVD. Moreover, attenuation of the vPI by the carotid siphon correlated negatively with iICAC volume and age.
Our study provides additional information on local pulsatility and arterial distensibility changes along the ICA trajectory in controls and patients with cSVD. A pulsatile flow rate can be accommodated by either pulsation in blood-flow velocity (influencing vPI) or by expansion of the vessel wall (reflected in distensibility). In this study, vPI was used as outcome instead of flow pulsatility, because flow is directly dependent on area and velocity (flow = velocity × area). The inverse relation between vPI and distensibility and the initial increase in vPI along the ICA in both groups was consistent with previous 3 T 2D-flow MRI measurements.9 The similar distensibility between the groups for the C3 and C4 segments might be related to effects from the bony canal on the distensibility at these locations.
Several studies have investigated arterial distensibility in the common carotid artery (CCA), but not distensibility along the whole ICA. Reported ultrasound CCA distensibility values in normal controls,19, 26, 27 range between 11.7 × 10−3 kPa−1 (mean 50 years)26 and 43.3 × 10−3 kPa−1 (mean 36 years)27 and 25 × 10−3 kPa−1 in an older population cohort in which cases with cSVD might be present (65–80 years).28 This wide range is possibly explained by differences in age, blood pressure, and measurement techniques. MRI distensibility measurements of the middle cerebral artery (MCA) have yielded values of approximately 4.4 × 10−3 kPa−1 (mean 30 years).14 This is much lower than the values we measure in the ICA, but this is consistent with decreasing distensibility as diameters decrease. Our ICA distensibility in controls (mean of all segments: 25 × 10−3 kPa−1) lies in the range of reported distensibility for the CCA. Our values are lower than the distensibility reported for the ICA (35.6 ± 13.2 × 10−3 kPa−1) in a small group of five healthy subjects (age range: 26–34) as reported by Canton et al.29 This is consistent with the considerably higher age of our controls.
The arterial diameter decreased from C1 toward C7 and was higher in controls compared to patients with cSVD. It is interesting to note that the differences in lumen diameter were largest at the C3 and C4 segments, where the vessel is constricted by the carotid canal. It seems plausible that vessel wall remodeling may have more effect on the lumen at C3/C4 than in the other segments where the surroundings allow for compensatory enlargement (positive remodeling).30, 31 The mean blood-flow in the ICA in this study matches that found in the literature.32 The lower mean blood-flow in patients with cSVD is in line with previous literature showing a relation between vessel stiffness (measured in the aorta) and cerebral blood flow.33
Variable flow rates could lead to intensity fluctuations and, subsequently, to apparent area changes. We believe that in practice this effect is very limited for the following reasons. First, we obtain similar observations for 2D and 4D phase-contrast acquisitions, while both acquisitions are sensitive to different inflow effects.9, 34 Second, we see very consistent variability in area pulsatility along the carotid canal (including lower pulsatility inside the bony carotid canal), which is inversely proportional to the velocity pulsatility. This is consistent with preservation of flow volume (area × velocity), but not with velocity-induced signal fluctuations. In the latter case, a higher velocity pulsatility index would rather yield a higher (apparent) area pulsatility.
Calcification results in increased arterial stiffness, as demonstrated previously with transcranial Doppler ultrasonography in the cerebral vasculature of ischemic stroke patients.35 Based on histology, iICAC in the carotid siphon appears to be more characteristic for vessel stiffness than for atherosclerotic plaque.10 Studies have shown that iICAC is associated with carotid artery stiffness,12 and with cognitive impairment and characteristic lesions of cSVD.36 By studying the difference in pulsatility between C1 and C7 in relation to the local iICAC, we largely eliminated effects of upstream vascular disease on the hemodynamics at the level of C1. Increased arterial stiffness is also associated with hypertension,12, 37, 38 and a larger volume of hyperintense white matter lesions in patients with cSVD,28 but not necessarily with lacunar infarcts or microbleeds.39 Arterial stiffness has also been studied with respect to cSVD, using alternative metrics to assess stiffness, such as brachial-ankle pulse wave velocity (PWV),40-42 and carotid-femoral PWV.39 Our finding that arterial stiffness is higher in the cSVD group compared to controls is in line with these studies.
We cannot say if iICAC is a cause or a consequence of cSVD, or a cause or consequence of disturbed hemodynamics. iICAC in patients with cSVD was associated with higher pulsatility and increased stiffness (decreased distensibility) along the ICA. This stiffening results in increased pulsatility in patients with cSVD instead of the expected vPI attenuation over the carotid siphon. Less vPI attenuation between C1 and C7 correlated with increasing calcification volume and/or age. Patients with cSVD without iICAC also had significantly higher vPI than the controls, confirming that iICAC is only one of the multifactorial contributors of increased vPI. The increase of vPI over the ICA in patients with cSVD confirms the association between higher intracranial vPI and cardiovascular disease, stroke, and vascular dementia due to damage to the microcirculation.43 The aorta and carotid artery stiffen at a similar rate with aging.44 Aortic PWV did not correlate with cerebral arterial pulsatility in controls in one study,6 but higher blood pressure levels, flow pulsatility and carotid-femoral PWV were associated with diffuse microvascular brain lesions and reduced cognition in another study.45 The increased pulsatile flow in patients with cSVD together with absent vPI attenuation supports the pulse wave encephalopathy theory, since it implies that a higher pulsatile flow reaches distal arterial segments, where it increases endothelial shear stresses. Interestingly, the mean blood-flow was lower in the patients. Assuming steady capillary flow, this implies less blood volume pulsations in the tissue, that is, less radial stretch of the arterial vessels embedded in the tissue. Given the notion that vessel wall pulsations are important for brain clearance,46 the reduced stroke volume could indicate reduced “vascular energy” for driving clearance. Further research is required in larger populations to test if, and how, increased ICA vPI might lead to increased cSVD-related brain tissue damage.
Limitations
First, the number of patients was limited and results need to be confirmed in a larger prospective study. Second, the limited FOV reduced statistical power at the extracranial segment (C1). Third, separate acquisition for each direction of velocity encoding was used, rather than in a single interleaved acquisition. Fourth, iICAC was only available in patients with cSVD. Lastly, significantly more subjects with hypertension were observed in the cSVD group compared to controls. Both systolic and diastolic pressures were not statistically significantly different between the two groups, which indicates that the hypertension is probably under control. However, we cannot rule out the possibility that past hypertension may have contributed to the differences in the cSVD group compared to the control group. Hence, the role of hypertension in carotid pulsatility attenuation in interplay with iICAC needs further investigation.
Conclusion
This study demonstrates that patients with cSVD have higher vPI and lower distensibility without effective vPI attenuation by the carotid siphon compared to controls. In patients with cSVD, iICAC is associated with increased vPI and decreased distensibility. These findings support the view that reduced pulsatility attenuation along the ICA is a potential contributor to cSVD and that iICAC may contribute to diminished pulsatility attenuation in patients with cSVD.
ACKNOWLEDGMENTS
The authors thank Jonas W. Bartstra for statistical advice.
Open Research
DATA AVAILABILITY
Anonymized data will be shared upon reasonable request to the corresponding author.




