The 1-13C galactose breath test in GALT deficient patients distinguishes NBS detected variant patients but does not predict outcome in classical phenotypes
Funding information: Metakids, Grant/Award Number: 2016-069; The AMC foundation
Abstract
Classical galactosemia (CG) patients frequently develop long-term complications despite early dietary treatment. The highly variable clinical outcome is poorly understood and a lack of prognostic biomarkers hampers individual prognostication and treatment. The aim of this study was to investigate the association between residual galactose oxidation capacity and clinical and biochemical outcomes in CG patients with varying geno- and phenotypes. The noninvasive 1-13C galactose breath test was used to assess whole body galactose oxidation capacity. Participants received a 7 mg/kg oral dose of 1-13C labelled galactose. The galactose oxidation capacity was determined by calculating the cumulative percentage dose of the administered galactose (CUMPCD) recovered as 13CO2 in exhaled air. Forty-one CG patients (5–47 years) and four adult controls were included. The median galactose oxidation capacity after 120 minutes (CUMPCDT120) of 34 classical patients (0.29; 0.08–7.51) was significantly lower when compared to two homozygous p.Ser135Leu patients (9.44; 8.66–10.22), one heterozygous p.Ser135Leu patient 18.59, four NBS detected variant patients (13.79; 12.73–14.87) and four controls (9.29; 8.94–10.02). There was a clear correlation between Gal-1-P levels and CUMPCDT120 (P < .0005). In the classical patients, the differences in CUMPCDT120 were small and did not distinguish between patients with poor and normal clinical outcomes. The galactose breath test distinguished classical patients from homo- and heterozygous p.Ser135Leu and NBS detected variant patients, but was not able to predict clinical outcomes in classical patients. Future studies are warranted to enable individualised prognostication and treatment, especially in NBS variants with galactose oxidation capacities in the control range.
Abbreviations
-
- CG
-
- classical galactosemia
-
- CUMPCD
-
- cumulative percentage of the administered dose recovered
-
- Gal-1-P
-
- galactose-1-phosphate
-
- GALT
-
- galactose-1-phosphate uridyltransferase
-
- IQ
-
- intelligence quotient
-
- MD
-
- movement disorder
1 INTRODUCTION
Classical galactosemia (CG, OMIM 230400) is an inborn error of galactose metabolism, caused by a deficiency of galactose-1-phosphate uridylyltransferase (GALT, EC 2.7.7.12). Severe illness in the newborn period is prevented by an early start of dietary treatment after newborn screening (NBS) or family screening but long-term complications such as cognitive impairment, movement disorders (MDs) and in females ovarian failure are frequently seen in both screened and symptomatically detected patients.1-7 The severity of long-term complications varies widely, even between siblings with identical mutations. Prognostic biomarkers are currently lacking, but are urgently needed for prognostication, especially in NBS detected patients with previously unknown genotypes and phenotypes8 and may facilitate individualised treatment.
The pathogenesis of CG and the cause of long-term complications is poorly understood. Endogenous galactose synthesis results in the persistent elevation of galactose-1-phosphate (Gal-1-P) which is presumed to be toxic and thought to competitively inhibit other pathways such as the production of uridine diphosphate glucose (UDP) sugars, which are essential for the synthesis of glycoproteins and glycolipids.2, 9, 10 Both accumulation of toxic metabolites and ongoing glycosylation abnormalities have been suggested to contribute to the long-term outcome in CG patients.11-13
In search for prognostic biomarkers, we hypothesize that differences in clinical outcome are caused by differences in residual GALT enzyme activity. A slightly higher residual enzyme activity may lead to a clinically relevant higher galactose oxidation capacity. This will cause lower Gal-1-P levels resulting in less abnormal galactosylation and possibly a more favourable clinical outcome. While measurement of erythrocyte GALT activity is the gold standard for diagnosis, the method used in our cohort is not able to reliably detect differences in enzyme activity below 3.3% (<1.1 umol/h.g Hb) and is therefore not suitable to study the correlation between residual enzyme activity and clinical outcome.
Previous studies showed that the non-invasive 13C galactose breath test reflects individual galactose oxidation capacity and was able to differentiate between galactosemia patients and controls,14-16 and even between GALT gene variations, such as the Duarte-variant (with erythrocyte GALT activity >15%) and p.Ser135Leu genotypes (with residual GALT activity in tissues other than erythrocytes).15 Although an association between galactose oxidation capacity and verbal dyspraxia has been found in CG patients,17 the association between residual galactose oxidation capacity and other clinical outcome measures, such as intellectual outcome and MDs have not been studied yet. Therefore, we determined the individual galactose oxidation capacity in our cohort of CG patients with different genotypes and phenotypes with the use of the 13C galactose breath test and investigated the association with clinical and biochemical outcomes.
2 METHODS
2.1 Participants
CG patients were invited to participate in this study by their treating physician. Participating patients were studied in the galactosemia expertise outpatient clinic of the Amsterdam UMC.
CG patients with two known pathogenic variations in the GALT gene and/or an erythrocyte GALT activity <15% of the reference mean were eligible for participation in this study. This cohort comprises patients with varying geno- and phenotypes such as patients with classical phenotypes (two pathogenic GALT mutations and absent or barely detectable erythrocyte GALT activity), NBS detected variant patients (detected since 2007 with previously unknown geno- and phenotypes8) and patients with the homo- and heterozygous p.Ser135Leu mutation.18 The control group consisted of parents of paediatric patients with a confirmed heterozygous mutation in the GALT gene. Patients with swallowing difficulties and/or patients unable to follow breath test instructions were excluded.
2.2 Clinical outcomes
In order to investigate the association between galactose oxidation capacity and clinical outcomes, patients were divided into subgroups based on their intellectual and neurological outcome. Intellectual outcome was defined as poor (IQ < 85) or normal (IQ ≥ 85) and neurological outcome was based on the presence or absence of MDs.
2.3 Biochemical outcomes
The most recent Gal-1-P level measured by gas chromatography mass spectrometry (GC-MS) was used in this study (usual range in diet adherent patients 0.05–0.82 μmol/g Hb). Patients with self-reported dietary incompliance at the most recent Gal-1-P measurement were excluded from the Gal-1-P analysis.
2.4 13-C breath test
The 1-13C1 labelled galactose was produced by Cambridge Isotope Laboratories, Inc.©, Massachusetts, United States. Analysis in our laboratory showed a singly labelled stable isotope with a purity of 99%, as expected.
To ensure a steady baseline of 13C abundance in breath CO2 over the study period, participants were instructed to eliminate 13C enriched products from their diet 2 days prior to the start of the 13C breath test19 and to keep a galactose restricted diet as specified in the international guideline20 for at least 24 hours prior to the start of the study. This prevented a decreased enrichment of supplemented 1-13C label with unlabelled galactose from the diet. A higher precursor enrichment (1-13C galactose) will lead to higher 13CO2 enrichment in breath. Participants were fasted for a minimum of 2 hours before start of the test and were only allowed to drink water during the test. During the test, participants maintained a resting state. All participants received a 7 mg/kg oral dose of 1-13C labelled galactose dissolved in water. Two baseline breath samples were collected before galactose ingestion. Patients were instructed to take a deep breath, to hold their breath for 3 seconds and to blow out into glass Vacutainer tube through a straw. Exactly 60, 90, and 120 minutes after the galactose ingestion, two breath samples were collected. Breath samples were stored at room temperature until analysis.
2.5 Measurement of 13CO2 in breath samples
In each breath sample, the enrichment of 13C in expired CO2 was measured by automated gas-isotope-ratio mass spectrometry on a GC-IRMS Delta XL plus system (Thermo Fisher, Bremen, Germany) using a PoraBOND Q column (25 m × 0.32 mm; Agilent Technologies, the Netherlands).21, 22 All collected samples were measured in triplicate. Standards and control samples were measured with each sequence of analyses. Results were expressed as the δ‰ vs the international reference standard, PeeDee Belemnite. Subsequently, CO2 production was calculated with the use of the Schofield equation, based on gender, age, height, and weight of participants and the Weir equation using a fasting respiratory quotient (RQ) of 0.80.23 The CO2 production and the isotopic enrichment values were used to calculate the percentage dose recovered per participant at each time point and with these values the cumulative percentage dose of 13C recovered from the labelled galactose (CUMPCD) as 13CO2 in exhaled air was calculated.24 The analytic variation was calculated for all participants.
2.6 Statistical analysis
SPSS version 25 (SPSS Inc. Chicago, Illinois) was used to perform all statistical analyses. Descriptive analyses included median and ranges because of a non-normal distribution. To determine if there were statistically significant differences in CUMPCDT120 between patients with poor and normal clinical outcomes the Mann-Whitney U test was used. The Spearman's rank coefficient test was used to test for correlations and in case of a significant correlation, linear regression was performed. P values below .05 were considered statistically significant.
3 RESULTS
A total of 43 patients and four controls were included in this study. Two patients were excluded after being unable to follow breath test instructions due to their young age.
3.1 Demographics
The data of 41 patients, 19 males, and 22 females with a median age of 18.0 years (5–47) and four controls, one male and three females with a median age of 44 years (38–49) are reported (Table 1). Our cohort consisted of 34 patients with a classical phenotype, hereafter classical patients, four NBS detected variant patients, two patients with the homozygous p.Ser135Leu genotype and one heterozygous p.Ser135Leu/p.*380Argext*50 patient. The erythrocyte GALT enzyme activity was below the limit of quantitation of the enzyme assay (<3.3%; <1.1 umol/h.g Hb) in 32/38 patients and between 3.6% and 8.7% in 6/38 patients.
| Pt ID | Group | GALT_1/GALT_2 | GALT activity (%) | Age at diagnosis (days) | CUMPCD T120 | IQ | MD |
|---|---|---|---|---|---|---|---|
| 1 | Cl | p.Gln188Arg/p.Gln188Arg | <3.3 | 6 | 0.29 | 64 | No |
| 2 | Cl | p.Gln188Arg/p.Gln188Arg | <3.3 | 6 | 1.92 | 89 | No |
| 3 | Cl | p.Gln188Arg/p.Gln188Arg | <3.3 | 11 | 0.48 | 64 | No |
| 4 | Cl | p.Gln188Arg/p.Gln188Arg | - | 8 | 0.25 | 91 | - |
| 5 | Cl | p.Gln188Arg/p.Gln188Arg | - | 10 | 0.22 | 71 | - |
| 6a | Cl | p.Gln188Arg/p.Gln188Arg | <3.3 | 0 (FS) | 0.17 | 61 | Yes |
| 7 | Cl | p.Gln188Arg/p.Gln188Arg | <3.3 | 10 | 0.23 | 82 | No |
| 8b | Cl | p.Gln188Arg/p.Gln188Arg | <3.3 | 0 (FS) | 0.12 | 71 | - |
| 9 | Cl | p.Gln188Arg/p.Gln188Arg | - | 9 | 0.24 | 53 | No |
| 10 | Cl | p.Gln188Arg/p.Gln188Arg | <3.3 | 8 | 0.32 | 83 | - |
| 11 | Cl | p.Gln188Arg/p.Gln188Arg | <3.3 | 14 | 0.29 | 45 | Yes |
| 12a | Cl | p.Gln188Arg/p.Gln188Arg | <3.3 | - | 0.21 | 81 | Yes |
| 13b | Cl | p.Gln188Arg/p.Gln188Arg | <3.3 | 0 (FS) | 0.12 | 77 | Yes |
| 14 | Cl | p.Gln188Arg/p.Gln188Arg | <3.3 | 14 | 0.08 | 78 | - |
| 15 | Cl | p.Gln188Arg/p.Leu195Pro | <3.3 | 9 | 4.59 | 82 | No |
| 16 | Cl | p.Gln188Arg/p.Leu195Pro | <3.3 | 10 | 0.28 | 103 | Yes |
| 17c | Cl | p.Gln188Arg/p.Leu195Pro | <3.3 | 0 (FS) | 0.43 | 52 | Yes |
| 18 | Cl | p.Gln188Arg/p.Leu195Pro | <3.3 | 18 | 0.14 | 93 | No |
| 19c | Cl | p.Gln188Arg/p.Leu195Pro | <3.3 | 7 | 0.30 | 88 | Yes |
| 20 | Cl | p.Gln188Arg/p.Ser135Trp | 3.9 | 8 | 0.13 | 65 | Yes |
| 21 | Cl | p.Gln188Arg/p.Ser135Trp | <3.3 | 29 | 0.19 | 98 | No |
| 22 | Cl | p.Gln188Arg/p.Ser135Trp | <3.3 | 46 | 0.35 | 57 | - |
| 23 | Cl | p.Gln188Arg/p.Ser135Trp | <3.3 | - | 0.59 | >85 | - |
| 24 | Cl | p.Gln188Arg/p.Lys285Asn | <3.3 | 22 | 0.15 | 49 | Yes |
| 25d | Cl | p.Gln188Arg/p.Lys285Asn | <3.3 | 24 | 1.04 | 76 | Yes |
| 26d | Cl | p.Gln188Arg/p.Lys285Asn | <3.3 | 24 | 2.02 | 86 | Yes |
| 27 | Cl | p.Gln188Arg/p.Lys285Asn | <3.3 | - | 0.24 | 77 | - |
| 28e | Cl | p.Arg148Gln/p.Trp316* | <3.3 | 0 (FS) | 0.53 | 68 | Yes |
| 29e | Cl | p.Arg148Gln/p.Trp316* | <3.3 | 14 | 0.67 | 88 | Yes |
| 30 | Cl | p.Gln188Arg/p.Lys127E | <3.3 | 0 (FS) | 0.96 | 61 | - |
| 31 | Cl | p.Gln188Arg/p.Lys127E | <3.3 | - | 0.37 | 70 | - |
| 32f | Cl | p.Gln188Arg/c.377+7A>C | <3.3 | 0 (FS) | 0.29 | 95 | - |
| 33f | Cl | p.Gln188Arg/c.377+7A>C | <3.3 | 14 | 0.50 | 87 | No |
| 34 | Cl | p.Ser135Trp/p.Arg51Gln | <3.3 | 10 | 7.51 | 78 | - |
| 35 | S | p.Ser135Leu/p.*380Rext*50 | <3.3 | 7 | 18.59 | 95 | No |
| 36 | S | p.Ser135Leu/p.Ser135Leu | 3.9 | 3860 | 10.22 | 61 | No |
| 37 | S | p.Ser135Leu/p.Ser135Leu | <3.3 | 210 | 8.66 | 71 | No |
| 38 | V | p.Gln188Arg/p.Met219Lys | 7.2 | 7 | 12.97 | 96 | No |
| 39 | V | p.Gln188Arg/c.1-96T>G | 3.6 | 8 | 12.73 | 86 | No |
| 40g | V | p.Val128Ile/p.Val128Ile | 8.7 | 9 | 14.87 | 86 | No |
| 41g | V | p.Val128Ile/p.Val128Ile | 8.7 | 9 | 14.61 | 89 | No |
| 42 | Co | c.1-96T>G/- | - | 9.27 | - | - | |
| 43 | Co | p.Gln188Arg/- | - | 10.02 | - | - | |
| 44 | Co | p.Gln188Arg/- | - | 8.94 | - | - | |
| 45 | Co | p.Ser135Trp/- | - | 9.32 | - | - |
- Notes: a,b,c,d,e,f,g: sibs, -: missing data. Patients are listed on genotype and increasing age; bold entries represents paediatric patients.
- Abbreviations: Cl, classical; Co, controls; FS, family screening; IQ, intelligence quotient; MD, movement disorder; S: homo- and heterozygous p.Ser135Leu; V, variants.
3.2 CUMPCDT120
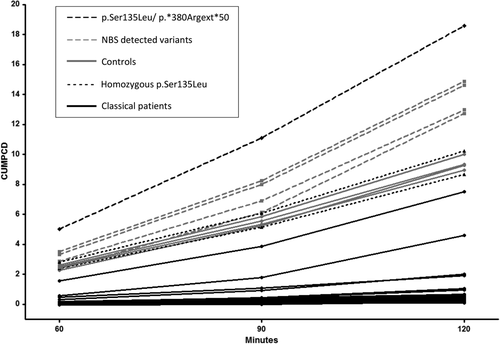
The CUMPCD levels of the participants are summarised in Table 2 and illustrated in Figure 1. The CUMPCD at 120 minutes (CUMPCDT120) of classical patients was significantly lower than of the NBS variants and of the homozygous p.Ser135Leu patients (P < .019). The CUMPCDT120 was significantly higher in paediatric patients when compared to adult patients (P = .001), which was also demonstrated in the classical paediatric patients (P = .019). The CUMPCDT120 did not differ between males and females.
| N | CUMPCD T60 | CUMPCD T90 | CUMPCD T120 | |
|---|---|---|---|---|
| All patients | 41 | 0.074 (−0.04−5.00) | 0.19 (−0.006−11.09) | 0.35 (0.08−18.59) |
| Controls | 4 | 2.49 (2.26−2.62) | 5.45 (5.23−5.84) | 9.29 (8.94−10.02) |
Classical patients Paediatric patients Adult patients |
34 14 20 |
0.06 (−0.04−1.54) 0.10 (−0.04−0.58) 0.05 (−0.03−1.54) |
0.16 (−0.006−3.87) 0.24 (−0.006−1.79) 0.13 (0.006−3.87) |
0.29 (0.08−7.51) 0.49 (0.13−4.59) 0.24 (0.08−7.51) |
| NBS detected variant patients | 4 | 3.08 (2.33−3.49) | 7.44 (6.12−8.25) | 13.79 (12.73−14.87) |
| Homozygous p.Ser135Leu | 2 | 2.59 (2.39−2.79) | 5.60 (5.14−6.07) | 9.44 (8.66−10.22) |
| p.Ser135Leu/ p.*380Argext*50 | 1 | 5.00 | 11.09 | 18.59 |
- Notes: Data reported in median and ranges.
- Abbreviation: CUMPCD, cumulative percentage dose recovered.
3.3 Correlation with age
Since the CUMPCDT120 was significantly higher in paediatric patients than in adult patients, the correlation between age and CUMPCDT120 was investigated in the largest group of patients with the same genotype, the homozygous p.Gln188Arg patients. This group consists of 14 classical patients with a median age of 23 years (6–35). Linear regression indicated a negative correlation between age and CUMPCDT120: F(1,12)5.77, β-0.30(95%CI −0,057 – −0,003), P = .033.
3.4 Analytic variation
Since the galactose oxidation capacity of classical patients was found to be within a narrow range, the analytic variability of the galactose oxidation test was assessed. The effect of the analytic variation varied between patients with a low and high CUMPCDT120, and was ±0.06 in the patient with the lowest CUMPCDT120 of 0.08 and ±0.16 in the patient with the highest CUMPCDT120 of 18.59.
3.4.1 The association between CUMPCDT120 and clinical outcomes
The CUMPCDT120 analyses were performed for paediatric and adult patients separately (Table 3). The two included p.Ser135Leu homozygous patients were diagnosed late (7 months and 10 years), which may affect their clinical outcome. Therefore, analyses were carried out both with and without these patients. The youngest patient in our cohort with a p.Ser135Leu/ p.*380Argext*50 genotype demonstrated the highest CUMPCDT120 of 18.59 and seems an outlier. To evaluate the influence of this result, analysis were performed with and without this patient.
| All patients | Classical patients | NBS variant patients | Homozygous p.Ser135Leu | p.Ser135Leu/p.*380Argext*50 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median age (years) | N 41 |
18.0 (5−47) | N 34 |
19.5 (6−47) | N 4 |
6.5 (5−8) | N 2 |
19.5 (16−23) | N 1 |
5.0 |
| Median CUMPCD T120 | 0.35 (0.08−18.59) | 0.29 (0.08−7.51) | 13.79 (12.73−14.87) | 9.44 (8.66−10.22) | 18.59 | |||||
| Median CUMPCD T120 paediatric patients | ||||||||||
IQ ≥ 85: IQ < 85: |
12 8 |
3.30 (0.28−18.59) 0.45 (0.13−10.22) |
7 7 |
0.67 (0.28−4.59) 0.43 (0.13−1.04) |
4 - |
13.79 (12.73−14.87) | - 1 |
10.22 |
1 - |
18.59 |
MDs, No: MDs, Yes: |
11 8 |
10.22 (0.29−18.59) 0.48 (0.13−2.02) |
5 8 |
0.50 (0.29−4.59) 0.48 (0.13−2.02) |
4 - |
13.79 (12.73−14.87) | 1 - |
10.22 |
1 - |
18.59 |
| Median CUMPCD T120 adult patients | ||||||||||
IQ ≥ 85: IQ < 85: |
5 16 |
0.25 (0.14−0.59) 0.24 (0.08−8.66) |
5 15 |
0.25 (0.14−0.59) 0.24 (0.08−7.51) |
- |
- 1 |
8.66 |
- | ||
MDs, No: MDs, Yes: |
5 5 |
0.23 (0.14−8.66) 0.21 (0.12−0.30) |
4 5 |
0.21 (0.14−0.24) 0.21 (0.12−0.30) |
- |
1 - |
8.66 | - | ||
- Abbreviations: CUMPCD, cumulative percentage dose recovered; IQ, intelligence quotient; MD, movement disorder; NBS, newborn screening.
3.5 Intellectual outcome
In total, 8/20 paediatric patients had a poor and 12/20 had a normal intellectual outcome. Paediatric patients with a normal intellectual outcome demonstrated a significantly higher CUMPCDT120 than patients with a poor intellectual outcome (P = .031). The difference remained significant after the exclusion of the homozygous p.Ser135Leu patient and the p.Ser135Leu/p.*380Argext*50 patient. For the classical paediatric patients, there was no significant difference in CUMPCDT120 between patients with a poor and normal intellectual outcome.
In total, 16/21 adult patients had a poor and 5/21 had a normal intellectual outcome. There was no significant difference in CUMPCDT120 between adult patients with a poor and normal intellectual outcome. This result remained unchanged after the exclusion of the homozygous p.Ser135Leu patient.
In both paediatric and adult patients, there was no significant correlation between the IQ (as continuous measure) and CUMPCDT120.
3.6 Neurological outcome
An MD was found in 8/19 paediatric patients and in 5/10 adult patients. The CUMPCDT120 in paediatric patients without an MDs was significantly higher when compared to paediatric patients with an MD (P = .013). The difference remained significant after the exclusion of the homozygous p.Ser135Leu patient and the p.Ser135Leu/ p.*380Argext*50 patient. For the classical paediatric patients, there was no significant difference in CUMPCDT120 between patients with and without an MD. There was no significant difference in CUMPCDT120 between adult patients with and without an MD. These results remained unchanged after the exclusion of the homozygous p.Ser135Leu patient.
3.7 Siblings
Our cohort includes seven sibling pairs and their data is summarised in Table S1.
3.7.1 The association between CUMPCDT120 and biochemical outcome
In 37/41 patients, the most recent Gal-1-P level measured by GC-MS was available. Linear regression indicated a negative correlation between the CUMPCDT120 and Gal-1-P: F(1,36)33.43, β-0.21 (95%CI −0,029 − −0,014), P < .0005. In the classical patients there was no significant correlation between CUMPCDT120 and Gal-1-P.
4 DISCUSSION
The results of our study indicate that the 13C breath test reflects individual galactose oxidation capacity by clearly distinguishing patients with classical phenotypes from NBS variants with higher erythrocyte GALT activity (up to 10%), and from homozygous p.Ser135Leu patients with deficient GALT activity in erythrocytes, but residual GALT activity in other tissues.
The results of most classical patients (CUMPCDT120≤2) and of the homozygous p.Ser135Leu patients (CUMPCDT120 in the same range as controls) were in line with previous research.15 Since previous research demonstrated comparable galactose oxidation capacity between individuals heterozygous for p.Gln188Arg and healthy controls, we included heterozygous parents as controls.25
On a group level, paediatric patients with a normal intellectual outcome and without MDs demonstrated a significantly higher galactose oxidation capacity than paediatric patients with a poor intellectual outcome and with MDs. This difference is caused by the inclusion of four NBS detected variant patients in the paediatric cohort, whose galactose oxidation capacity even exceeded those of the controls. This might be due to their younger age, as we found a significant correlation between age and galactose oxidation capacity in patients with an identical genotype (homozygous p.Gln188Arg). Previous research also demonstrated that galactose oxidation capacity fluctuates in the first weeks of life14 and in an animal model, age-dependent GALT activity was demonstrated in different tissues.26 At this moment, it is unclear if younger patients truly have a higher galactose oxidation capacity or whether other factors that vary with age have influenced the breath test results, such as body composition, temporary label trapping22 or differences in alternative disposal pathways, such as upregulation of the UDP-glucose pyrophosphorylase pathway.
The negative correlation found between the patients' CUMPCDT120 and Gal-1-P levels indicates that higher galactose oxidation capacity results in lower Gal-1-P levels. This is mainly the effect of the four NBS variant patients with higher galactose oxidation capacity and lower Gal-1-P levels than classical patients. Even though the NBS variant patients are young, currently none of them demonstrate long-term complications, which seems to support our hypothesis that differences in clinical outcome are caused by differences in residual galactose oxidation capacity. In the classical patients however, both CUMPCDT120 and Gal-1-P levels were within a narrow range and no significant differences in galactose oxidation capacity between patients with poor and normal clinical outcomes were demonstrated. This suggests that the differences in clinical outcomes in this subgroup may be attributed to other factors than differences in (residual) whole body galactose oxidation capacity.
In contrast to previous research in which all classical patients demonstrated CUMPCDT120 values <2,15 two classical patients in our study had a CUMPCDT120>2. These patients have erythrocyte GALT activity below the limit of quantitation and demonstrated CG related illness at diagnosis. Since one patient is young with a classical p.GlnQ188Arg/p.Leu195Pro genotype and a normal clinical outcome and the other is an older patient with a p.Ser135Thr/p.Arg51Gln genotype and a poor intellectual outcome, their results might be attributed to both age and genotype. This may also be the case for the youngest patient in our cohort with a p.Ser135Leu/p.*380Rext*50 genotype who has the highest CUMPDT120, despite an erythrocyte GALT activity below the limit of quantitation. This NBS detected patient demonstrated only hyperbilirubinemia at diagnosis and currently demonstrates no long-term complications. The homozygous p.Ser135Leu patients in our cohort have a CUMPCDT120 in the control range without MDs, but both have a poor intellectual outcome. Their poor intellectual outcome may well be caused by their late initiation of dietary treatment. Since the early treated NBS variant patients and the p.Ser135Leu/p.*380Rext*50 patient all have a CUMPCDT120 in the control range without demonstrating long-term complications, the question remains whether a CUMPCDT120 above 9 could be associated with a better clinical outcome if patients are treated early.
4.1 Limitations
This cohort includes 41 patients with varying ages and genotypes and the necessity to analyse results separately for adults and paediatric patients resulted in an even smaller sample size and hampered statistical power. To minimise the burdensome fasting and resting state in children in particular, and because previous research demonstrated a peak release of 13CO2 into expired air at 120 minutes, we decided to measure the galactose oxidation capacity until 120 minutes.25 After analysing the results, a majority of the patients showed flattening in the area under the curve at 120 minutes, but not all patients had reached their maximum oxidation capacity at 120 minutes yet and this might have influenced the results.
The negative correlation we found between age and galactose oxidation capacity in patients with an identical genotype poses a limitation to the use of this test. Further studies are warranted to investigate whether this is truly due to a higher galactose oxidation capacity at a younger age.
4.2 Strengths
For the rarity of the disorder, we included a relatively large number of patients that represent the full genetic, biochemical, and clinical outcome spectrum of CG. The inclusion of multiple sibling pairs with a different intellectual outcome in some siblings, and the inclusion of twins provided more insight into whole body galactose oxidation.
To ensure NBS variant and p.Ser135Leu patients did not influence the results, analyses were performed both with and without these patients.
4.3 Future perspectives
In our cohort, the galactose oxidation capacity of the classical patients was within a narrow range and some differences between patients may be attributed to analytic variation. Also, sibling pairs with different clinical outcomes did not demonstrate significant differences in oxidation capacity. The limited number of patients may have affected the results or the 1-13C breath test may not be sensitive enough to discriminate between classical patients.
Even though differences were small, the galactose oxidation capacity did differ between classical patients with identical genotypes. The use of universally 6-13C labelled galactose might be able to differentiate within the group of classical patients by providing larger differences in 13CO2 enrichment and thus enable better distinction between these patients.
In order to correctly interpret the results of the galactose oxidation breath test and to improve our understanding of galactose metabolism, the effects of age, body composition and genotype on galactose oxidation capacity should be further investigated in a larger cohort for which international cooperation will be necessary. Also, repeating the 13C galactose breath test in the same patient cohort might provide valuable information with regard to the course of the galactose oxidation capacity with increasing age.
We demonstrated that NBS detects a group of variant patients with erythrocyte GALT activity up to 10%, with significantly higher galactose oxidation capacity than the classical patients, and no long-term complications so far. The question remains whether these patients are at risk for long-term complications or might even benefit from a less strict diet. Future studies addressing the galactose tolerance of these individuals are warranted to prevent overtreatment.
5 CONCLUSIONS
The 13C galactose oxidation breath test is able to distinguish between classical patients, homozygous p.Ser135Leu patients and NBS variant patients, but is not able to differentiate between classical patients with poor and normal clinical outcomes in our cohort. Since NBS variant patients demonstrated galactose oxidation capacities in the control range, future studies are warranted to enable individualised prognostication and treatment, especially in these patients.
ACKNOWLEDGMENTS
The authors would like to thank Diana Ruffato Resende Campanholi for providing insight into the galactose oxidation calculations used in the study “Galactose oxidation using 13C in healthy and galactosemic children”.
CONFLICT OF INTEREST
Mendy M. Welsink-Karssies, Dewi van Harskamp, Sacha Ferdinandusse, Hidde H. Huidekoper, Mirian C.H. Janssen, E. Marleen Kemper, Janneke G. Langendonk, M. Estela Rubio Gozalbo, Maaike C. de Vries, Frits A. Wijburg and Henk Schierbeek declare that they have no conflict of interest. Carla E.M. Hollak is involved in premarketing studies with Sanofi, Protalix and Idorsia in the field of lysosomal storage disorders. She reports no conflicts of interest in relation to the current study. Annet M. Bosch received a speakers fee from Nutricia and was member of the advisory board of Biomarin.
AUTHOR CONTRIBUTIONS
M.M.W.-K. contributed to the design of the study, the data collection, data analysis and interpretation, drafted the initial manuscript, and critically revised the manuscript. D.H. and H.S. contributed by measuring the purity of the labelled galactose, contributed to the data analysis and interpretation, and critically reviewed the manuscript. S.F. measured the Gal-1-P levels and critically reviewed the manuscript. E.M.K. contributed to the storage, preparation and distribution of the labelled galactose and critically reviewed the manuscript. C.E.M.H., H.H.H., M.C.H.J., J.G.L., E.R.-G. and M.C.V. contributed to the data collection, and critically reviewed the manuscript. F.A.W. contributed to the interpretation of the data and critically reviewed the manuscript. A.M.B. contributed to the funding of the study, the design of the study, the data collection, data analysis and interpretation, drafted the initial manuscript, and critically revised the manuscript. All authors approved the final manuscript as submitted.
ETHICS STATEMENT
This study was approved by the Medical Ethical Committee of the Amsterdam University Medical Center.
INFORMED CONSENT
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975. Before study enrolment, informed consent was obtained from all participants, their parents or their legal representatives and all participants consented with the use of their data for research purposes.
ANIMAL RIGHTS
This article does not contain any studies with animal subjects performed by any of the authors.




