Effects of enzymes on ester production during the course of a Chinese liquor fermentation as discussed by correlation analysis and path analysis
Abstract
During the course of a Chinese liquor fermentation, the enzyme activities in the fermenting grains have important effects on ester production. Activities of six kinds of major hydrolases (including amylase, glucoamylase, protease, cellulase, pectinase and lipase) and the production of esters (including total esters, ethyl acetate, ethyl butyrate, ethyl caproate and ethyl lactate) were measured in the fermenting grains. The correlation coefficient between enzyme activities and ester production was calculated through correlation analysis, and the direct and indirect path coefficients of enzyme activities on ester production were obtained by path analysis. The results showed that enzymes had not only direct effects, but also indirect effects via other enzymes on ester production, indicating that the origin and composition of the enzyme effects on ester production were specific. Copyright © 2014 The Institute of Brewing & Distilling
Introduction
The production of Chinese liquor is a process involving anaerobic fermentation. Esters, which comprise about 75–95% of the total flavour substances, are the main flavour substance in Chinese liquor 1. There are about 100 kinds of esters in Chinese liquor among which ethyl acetate, ethyl butyrate, ethyl caproate and ethyl lactate are the four main esters and comprise about 90% of the total ester content 2. Flavour compounds are essential to liquor aroma, taste and style 3. For example, ethyl caproate can confer a fruit flavour to the liquor 4. In addition to the presence of esters in the raw materials, a large number of esters are produced by microbes during the fermentation of the grains 5. In general, flavour compounds are mainly produced, but are not limited to fermentation 6.
Synthesis of esters can be affected by many factors during fermentation. The composition of the fermentation medium can affect the amount of formation of esters 7, for example, addition of amino acids or ammonium salt can enhance the content of total esters in wine 8. Based on the report by Erten et al. 9, the concentration of isoamyl acetate was reduced with an increased pitching rate in high-gravity brewing. Secondly, microorganisms present in the fermenting grains and the enzymes secreted by them have an impact on ester production. During fermentation, enzymes produced by microorganisms in the fermenting grain break down high-molecular-weight materials such as proteins, carbohydrates, and fats, into low-molecular-weight substances such as amino acids, oligosaccharides and fatty acids, respectively. The small molecules are utilized as nutrients by the microorganisms, and they provide abundant precursors for flavour substances. The enzyme activities not only influence the transformation of various substances in fermenting grains, but also directly affect the species and quantity of flavour substances, which have a profound influence on the liquor 10. For example, esterase directly catalyses the esterification reaction of acetic acid and propanoic acid with ethanol to synthesize acetic ether and ethyl propionate during fermentation 11. According to the report by Wu et al. 12, increasing the lipase activity in fermenting grains could increase the total ester content in liquor. Adding cellulase into fermenting grains could improve the ester content, particularly ethyl acetate 13, 14.
Microorganisms including moulds, actinomycetes, yeast and bacteria in fermenting grains come from the Koji, brewing water, air and pits. Because Chinese liquor fermentation is an open system, microorganisms in the fermenting grains are very variable owing to the differences in the natural environment 15. Different microorganisms may have different fermentation capacities, thus the flavour compounds in Chinese liquor in different locations can vary widely. The special role of microorganisms will affect the composition of the enzyme systems in the fermenting grains, thus affecting the formation of esters 16. According to Xin et al. 17, when the amount of Propionibacteria added into the fermenting grains was 6.7 × 105 cells/g, the ethyl lactate content in liquor was reduced by 80.6 mg/100 mL, and the ethyl propionate content was increased by 0.5 mg/100 mL, compared with the controls. Zeng et al. 18 studied the effects of the addition of Bacillus on the flavour of liquor, and the results showed that the Bacillus could effectively improve the content of total acids, total esters and other flavour substances in the liquor. The addition of Monascus to fermenting grains was beneficial for the growth in content of total esters in the liquor 19. Monascus can metabolize esterified enzymes, which can further increase the amount of ethyl caproate 20.
The effect of the microorganisms on the esters can be achieved by changing the enzyme activities. In order to explore the relationship between enzyme activities and ester production, the activities of the enzymes (including α-amylase, glucoamylase, protease, cellulase, pectinase and lipase) and the production of esters (including total ester, ethyl acetate, ethyl butyrate, ethyl caproate and ethyl lactate) in fermenting grains was measured throughout the course of the fermentation. The correlation coefficients between enzyme activities and ester production were calculated, and the source and formation of the correlations were studied through path analysis.
Materials and methods
Production of grains for fermentation
Wheat flour (800 g) and sorghum flour (800 g) were mixed with 1000 mL of water preheated to 80°C, and placed in a thermal insulation box. After 18 h, 300 g of rice husk was added and mixed. The beaker was heated using steam for 80 min. After the temperature of the sample had decreased to 25°C, 200 g of Daqu powder was added and mixed. The beaker was sealed and placed in an incubator for anaerobic fermentation at 25°C.
Determination of enzyme activity
Fermenting grains (10 g), to which 20 mL of water was added, were sampled daily from the second day of fermentation. The samples were kept on a shaker for 30 min, and centrifuged at 4000 rpm. The enzyme activity of the supernatant was measured and converted into enzyme activity in the fermenting grains.
The α-amylase activity was determined by the method reported previously 21. Glucoamylase activity was determined by the DNS(3,5-dinitrosalicylic acid) method 22. Protease activity was measured by the Folin–Phenol method 23. Cellulase activity (FPase activity) was measured as described by Ghose 24.
To measure pectinase activity, 0.9 mL of a 0.25% pectin solution (in 0.5 mol/ L, pH 5. 0 acetate buffer) was first incubated at 40°C for 5 min, then 0.1 mL of enzyme was added and incubated for 30 min. An aliquot (1 mL) of 3,5-dinitrosalicylic acid reagent was added, and boiled for 5 min, cooled immediately; 4 mL of water was added and absorbance was read at 520 nm. One unit of enzyme was defined as the amount of enzyme producing 1 mmol galacturonic acid per min. Lipase activity was measured according to method of Vorderwülbecke et al. 25.
Determination of ester content
Fermenting grains (20 g) were taken daily from the second day of fermentation for distillation and 50 mL of water was added. Distillation was stopped when the distillate reached 40 mL. The ester content of the distillate was measured and converted into ester content of the fermenting grain.
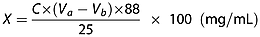 (1)
(1)The contents of ethyl acetate, ethyl butyrate, ethyl caproate and ethyl lactate were detected using a Siemens (Germany) GC L 350 gas chromatograph 27. The chromatography conditions were as follows: detector, Flame Ionization Detector (FID); split, 1,18; injector temperature, 210°C; detector temperature, 210°C; temperature interval, 50–200°C; The minimum detection limit (DT)/°C/min, 5; column, DB-5, 60 m × 0.247 mm; carrier gas, N2, 30 mL/min; internal standard, 2-ethyl-1-hexanol; injection volume, 1 μL. Analysed samples were compared with standards by GC and their identity was confirmed by mass spectrometry (MS 455 Siemens, Germany).
Correlation analysis and path analysis
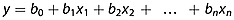 (2)
(2)The measured values are substituted into formula 2, and equations are built and solved by the principle of least squares, then the path coefficients (Pyxi) are obtained. The path coefficient is the standard partial regression coefficient of the variable, indicating the relative importance of the variables to the result.
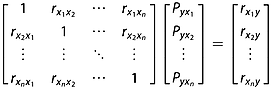 (3)
(3)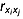 is the correlation coefficient between xi and xj,
is the correlation coefficient between xi and xj,  is the correlation coefficient between xi and y,
is the correlation coefficient between xi and y, 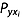 is the direct path coefficient of xi to y.
is the direct path coefficient of xi to y. 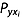 is obtained by solving eqn 3:
is obtained by solving eqn 3:
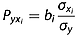 (4)
(4)In eqn 4, bi is the partial regression coefficient, 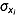 is the standard deviation of xi, σy is the standard deviation of y, and
is the standard deviation of xi, σy is the standard deviation of y, and 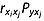 is the indirect path coefficient of xi via xj to y.
is the indirect path coefficient of xi via xj to y.
Results and discussion
From the second day of fermentation, samples were taken daily to determine the enzyme activities and the content of the esters. The average enzyme activities and the increase in the amount of esters on adjacent days were calculated. The results are shown in Table 1.
| Fermentation time (days) | Average enzyme activity (U/g) | Increment in ester content (mg/g) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Amylase | Glucoamylase | Protease | Cellulase | Pectinase | Lipase | Total ester | Ethyl acetate | Ethyl butyrate | Ethyl caproate | Ethyl lactate | |
| 2–3 | 11.56 | 12.48 | 20.0 | 10.85 | 14.35 | 7.55 | 0.09 | 0.008 | 0.002 | 0.002 | 0.071 |
| 3–4 | 10.74 | 15.05 | 20.95 | 13.57 | 15.32 | 8.7 | 0.07 | 0.013 | 0.003 | 0.013 | 0.058 |
| 4–5 | 10.50 | 17.75 | 25.2 | 15.65 | 17.9 | 10.95 | 0.04 | 0.008 | 0.004 | 0.008 | 0.033 |
| 5–6 | 10.75 | 19.15 | 47.87 | 22.7 | 14.85 | 13.1 | 0.11 | 0.046 | 0.005 | 0.006 | 0.036 |
| 6–7 | 11.85 | 20.58 | 31.45 | 16.8 | 10.9 | 14.25 | 0.12 | 0.073 | 0.007 | 0.009 | 0.041 |
| 7–8 | 12.45 | 22.72 | 33.55 | 14.55 | 12.05 | 15.05 | 0.13 | 0.062 | 0.002 | 0.003 | 0.058 |
| 8–9 | 11.85 | 17.85 | 29.25 | 13.16 | 11.75 | 12.58 | 0.09 | 0.014 | 0.002 | 0.005 | 0.074 |
| 9–10 | 10.05 | 14.15 | 21.3 | 12.10 | 9.15 | 11.88 | 0.09 | 0.017 | 0.001 | 0.009 | 0.062 |
| 10–11 | 10.45 | 12.84 | 17.68 | 11.62 | 8.34 | 11.7 | 0.08 | 0.015 | 0.002 | 0.001 | 0.051 |
| 11–12 | 9.75 | 11.62 | 15.36 | 10.42 | 5.98 | 11.05 | 0.04 | 0.013 | 0.001 | 0.001 | 0.017 |
| 12–13 | 9.46 | 10.54 | 10.82 | 8.94 | 3.15 | 10.2 | 0.08 | 0.042 | 0.002 | 0.001 | 0.033 |
| 13–14 | 8.04 | 9.42 | 5.70 | 5.67 | 1.5 | 9.45 | 0.06 | 0.021 | 0.001 | 0.001 | 0.029 |
The content of esters in the fermenting grains increased with the fermentation time (Table 1), but enzyme activities presented a complex change trend during the course of the fermentation. Enzyme activities were high in the early and mid period, while near the end, the enzyme activities decreased owing to a decline in microbial activity 30. The rate of increase of the ester content in the fermenting grains varied widely with the changes in the enzyme activities.
By correlation analysis, the correlation coefficients between enzyme activities and ester production were calculated. Meanwhile, the correlation coefficients between the different enzyme activities were obtained for path analysis. The results are shown in Table 2.
| Glucoamylase | Protease | Cellulase | Pectinase | Lipase | Total ester | Ethyl acetate | Ethyl butyrate | Ethyl caproate | Ethyl lactate | |
|---|---|---|---|---|---|---|---|---|---|---|
| Amylase | 0.805** | 0.691* | 0.578* | 0.675* | 0.484 | 0.657* | 0.359 | 0.425 | 0.292 | 0.619* |
| Glucoamylase | 0.863** | 0.793** | 0.647* | 0.772* | 0.645* | 0.587 | 0.624* | 0.456 | 0.272 | |
| Protease | 0.953** | 0.682* | 0.647* | 0.626* | 0.470 | 0.636* | 0.396 | 0.224 | ||
| Cellulase | 0.726* | 0.555 | 0.470 | 0.416 | 0.752** | 0.512 | 0.067 | |||
| Pectinase | 0.091 | 0.179 | −0.077 | 0.496 | 0.615* | 0.416 | ||||
| Lipase | 0.615* | 0.711** | 0.401 | 0.092 | −0.02 |
- ** Correlation is significant at the 0.01 level (two-tailed).
- * Correlation is significant at the 0.05 level (two-tailed).
It can be seen from Table 2 that the correlations between different enzyme activities and ester production showed a wide variation in values. Total ester production had a significant correlation with amylase, glucoamylase, protease and lipase. Ethyl acetate production had a significant correlation with lipase. Ethyl butyrate production had a significant correlation with glucoamylase, protease and cellulase. The correlation between ethyl caproate and pectinase was significant. Enzyme activity and the quantity of the precursors are two main factors in the production of esters 2. When the precursors are insufficient, ester production correlates with precursor. The different precursors have a different relationship with the hydrolytic enzymes 31, thus different correlations exist between different enzymes and esters. The enzymes directly affect the degradation of substances in the fermenting grains, and these substances provide nutrition to the microbial cells, thus affecting the secretion of enzymes, so different enzymes have different correlations. Some correlations between the enzymes are highly significant; for example, the correlation coefficient between protease and cellulase was 0.953.
The correlation coefficients only represent the closeness of the relationship between the enzymes and the esters. They cannot explain or analyse the composition and origin of this relationship. In addition, enzymes can affect each other mutually. Therefore in order to reveal the true relationship between enzyme activity and esters, the direct and indirect effects of enzymes on esters production were calculated using path analysis. The results of the path analysis are shown in Tables 3-7.
| Correlation coefficient | Direct effect | Indirect effect | ||||||
|---|---|---|---|---|---|---|---|---|
| Amylase | Glucoamylase | Protease | Cellulase | Pectinase | Lipase | |||
| Amylase | 0.657 | 0.691 | 0.634 | 0.545 | 0.039 | −0.888 | −0.365 | |
| Glucoamylase | 0.645 | 0.788 | 0.556 | 0.681 | 0.054 | −0.851 | −0.583 | |
| Protease | 0.626 | 0.789 | 0.477 | 0.680 | 0.065 | −0.897 | −0.488 | |
| Cellulase | 0.470 | 0.068 | 0.399 | 0.625 | 0.752 | −0.955 | −0.419 | |
| Pectinase | 0.179 | −1.315 | 0.418 | 0.510 | 0.538 | 0.049 | −0.069 | |
| Lipase | 0.615 | −0.755 | 0.334 | 0.608 | 0.510 | 0.038 | −0.120 | |
| Correlation coefficient | Direct effect | Indirect effect | ||||||
|---|---|---|---|---|---|---|---|---|
| Amylase | Glucoamylase | Protease | Cellulase | Pectinase | Lipase | |||
| Amylase | 0.359 | 0.435 | 1.666 | −0.674 | 0.984 | −1.464 | −0.588 | |
| Glucoamylase | 0.587 | 2.070 | 0.350 | −0.841 | 1.350 | −1.403 | −0.938 | |
| Protease | 0.470 | −0.975 | 0.301 | 1.786 | 1.622 | −1.479 | −0.786 | |
| Cellulase | 0.416 | 1.702 | 0.238 | 1.642 | −0.929 | −1.575 | −0.674 | |
| Pectinase | −0.077 | −2.169 | 0.294 | 1.339 | −0.665 | 0.321 | −0.111 | |
| Lipase | 0.711 | −1.215 | 0.211 | 1.598 | −0.631 | 0.945 | −0.197 | |
| Correlation coefficient | Direct effect | Indirect effect | ||||||
|---|---|---|---|---|---|---|---|---|
| Amylase | Glucoamylase | Protease | Cellulase | Pectinase | Lipase | |||
| Amylase | 0.292 | −0.268 | 1.018 | −0.940 | 0.706 | 0.048 | −0.271 | |
| Glucoamylase | 0.456 | 1.264 | −0.058 | −1.175 | 0.968 | 0.046 | −0.432 | |
| Protease | 0.396 | −1.361 | −0.185 | 1.091 | 1.164 | 0.048 | −0.362 | |
| Cellulase | 0.512 | 1.221 | −0.155 | 1.002 | −1.297 | 0.052 | −0.310 | |
| Pectinase | 0.615 | 0.071 | −0.181 | 0.818 | −0.928 | 0.886 | −0.051 | |
| Lipase | 0.092 | −0.559 | −0.130 | 0.976 | −0.881 | 0.678 | 0.006 | |
| Correlation coefficient | Direct effect | Indirect effect | ||||||
|---|---|---|---|---|---|---|---|---|
| Amylase | Glucoamylase | Protease | Cellulase | Pectinase | Lipase | |||
| Amylase | 0.425 | 0.381 | 1.228 | −1.384 | 1.609 | −0.880 | −0.529 | |
| Glucoamylase | 0.624 | 1.526 | 0.307 | −1.729 | 2.208 | −0.843 | −0.845 | |
| Protease | 0.636 | −2.003 | 0.263 | 1.317 | 2.653 | −0.889 | −0.708 | |
| Cellulase | 0.752 | 2.784 | 0.220 | 1.210 | −1.909 | −0.946 | −0.607 | |
| Pectinase | 0.496 | −1.303 | 0.257 | 0.987 | −1.366 | 2.021 | −0.100 | |
| Lipase | 0.401 | −1.094 | 0.184 | 1.178 | −1.296 | 1.545 | −0.119 | |
| Correlation coefficient | Direct effect | Indirect effect | ||||||
|---|---|---|---|---|---|---|---|---|
| Amylase | Glucoamylase | Protease | Cellulase | Pectinase | Lipase | |||
| Amylase | 0.619 | 0.610 | −0.554 | 1.039 | −1.018 | 0.464 | 0.076 | |
| Glucoamylase | 0.272 | −0.688 | 0.491 | 1.298 | −1.396 | 0.444 | 0.122 | |
| Protease | 0.224 | 1.504 | 0.422 | −0.594 | −1.678 | 0.469 | 0.102 | |
| Cellulase | 0.067 | −1.761 | 0.353 | −0.546 | 1.433 | 0.499 | 0.088 | |
| Pectinase | 0.416 | 0.687 | 0.412 | −0.445 | 1.026 | −1.278 | 0.014 | |
| Lipase | −0.02 | 0.158 | 0.295 | −0.531 | 0.973 | −0.977 | 0.063 | |
It can be seen from Tables 3-7 that the total effect of the enzymes on ester production includes direct effects and indirect effects, and the sum of the two effects is the correlation coefficient between enzymes and ester production. For example, the direct effect of cellulase on total ester production was 0.068, and the indirect effect via amylase, glucoamylase, protease, pectinase and lipase was 0.399, 0.625, 0.752, −0.955 and −0.419 respectively. The sum of these effect was 0.470 – the correlation coefficient between cellulase and total ester production.
The direct effect of the enzymes on ester production reflects the sole impact of the enzymes on ester production 32. Because enzymes have different roles during the fermentation of the grains, various enzymes have a different direct effect on ester production. For example, the effect of amylase, glucoamylase and protease on total ester production was 0.691, 0.788 and 0.789 respectively. The effect of cellulase was only 0.068, while the effect of pectinase and lipase was −1.315 and −0.755.
The indirect effect of one enzyme via other enzymes on ester production is associated with the direct effects of other enzymes on ester production and the correlation coefficients between one enzyme and other enzymes, which is the product of the correlation coefficient between the enzymes and the direct effect of the enzyme on the ester production. When one enzyme has some direct effect on ester production, other enzymes will have some indirect effect on ester production via this enzyme. For example, the direct effect of cellulase on total ester production is only 0.058, so the indirect effects of other enzymes on total ester production via cellulase are all <0.1. While the direct effect of one enzyme on ester production is very large, other enzymes have significant indirect effects on ester production via this enzyme. For example, the direct effect of cellulase on ethyl acetate production is 2.784, and the indirect effects of other enzymes via cellulase on the ethyl acetate production are all >1.
According to the data, the differences in correlation between the various enzymes and total ester production are very small, while the differences in correlation between various enzymes and the production of ethyl acetate, ethyl butyrate, ethyl caproate and ethyl lactate are very large. The reason for this is that the effects of enzymes on total ester production reflect the comprehensive effects of enzymes on the production of various ester. For example, cellulase apparently has a positive direct effect on the production of ethyl acetate, ethyl butyrate and ethyl caproate, but it has a negative direct effect on the production of ethyl lactate, which decreases the effect of cellulase on total ester production.
Fermenting grains for Chinese liquor is an open system during the fermentation stage. There is an exchange of energy and matter between this system and the external environment, and the system is far from equilibrium because of microbial metabolism. Based on the theory of dissipative structure, the system of liquor fermenting grains is a dissipative structure 33. In this system, various enzymes secreted by the microorganisms degrade raw materials for the growth and metabolism of microorganisms, so the enzyme activities are control parameters. According to the theory of dissipative structure, control parameters have a complex influence on the result 34. They are characterized by the complex correlation between enzyme activities and ester production in the fermenting grain system. Using path analysis, the correlation between enzyme activities and ester production can be explained as direct and indirect effects and so the effects of enzymes on ester production can be analysed and evaluated. During the fermentation of Chinese liquor, the enzyme activities can regulate the ester production not only by direct effect, but also by indirect effects via other enzymes.
Acknowledgements
This work was supported by a Fund of Qiqihar University Science and Technology research start-up support project for young teachers (2011 K-M35).




