Reactions of 4-dimethylamino-3-quinolinyl sulfides with nitrating mixture and transamination of 4-dimethylamino-3-methylsulfinyl-6-nitroquinoline
Abstract
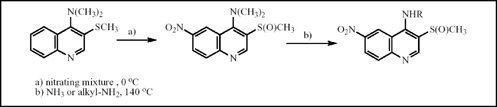
4-Amino-3-quinolinyl sulfides 4d-e and 7a-c were prepared by amination of 4-chloro-3-quinolinyl sulfides 4c or 1c, respectively, in methanol (140-160 °C) or in boiling phenol with yields up to 95 %. Reaction of 4-dimethylamino-3-quinolinyl sulfides 7c and 4e with nitrating mixture proceeded simultanously as oxidation of the methylthio group to the methylsulfinyl one and as C6-nitration to form 6-nitro-β-quinolinyl sulfoxides 9c or 10b, respectively. 4-Dimethylamino-3-methylsulfinyl-6-nitroquinoline 9c underwent acid catalysed transamination when reacting with primary aliphatic amines and ammonia.