Facile acidic hydrolysis and displacement reactions of 2-chloro-and 2,9-dichloro-1,10-phenanthroline
Abstract
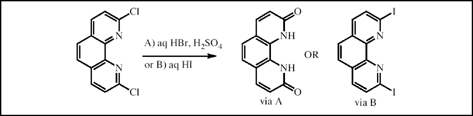
Treatment of 2-chloro- or 2,9-dichloro-1,10-phenanthroline with aqueous HBr or aqueous H2SO4 at 120°C yielded 1,10-phenanthroline-2(1H)-one or 1,10-dihydro-1,10-phenanthroline-2,9-dione, respectively. The hydrolysis of 2,9-dichloro-1,10-phenanthroline with 37% aqueous HC1 led to the half hydrolyzed amide and the bis-amide. Under comparable reactions conditions, using aqueous HBr, H2SO4 or HC1, 2-chloropyridine was found to be hydrolytically stable. On the other hand, 2-chloro- or 2,9-dichloro-1,10-phenanthroline on heating with 57% aqueous HI afforded the HI salts of 2-iodo- or 2,9-diiodo-1,10-phenanthroline, which could be isolated. These salts on treatment with aqueous ammonium hydroxide led to good yields of 2-iodo- and 2, 9-diiodo-1,10-phenanthroline, respectively. Treatment of 2-chloropyridine with 57% aqueous HI under similar reaction conditions led to 2-iodopyridine in a 10% conversion.