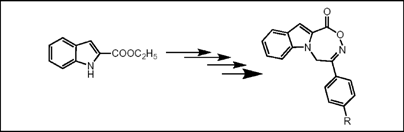
Facile synthesis of 6-aryl-5H-8-oxa-4b, 7-diaza-benzo[a]azulen-9-one derivatives, new tricyclic heterocycles
Abstract
8-Oxa-4b,7-diaza-benzo[a]azulene-9-one system, a new tricyclic heterocyclic framework is designed through a simple and convenient synthetic sequence. Its 6-aryl derivatives are synthesized starting from ethyl indole-2-carboxylate. Reaction of differently substituted phenacyl bromides with ethyl indole-2-carboxylate, treatment of the resultant N-substituted indole-2-carboxylates with hydroxylamine hydrochloride to provide corresponding oximes, subsequent ester hydrolysis followed by dehydrative cyclisation furnished the desired compounds 5 a-g.