Regioselective synthesis of heterocyclic scaffold by aryl radical cyclization
Abstract
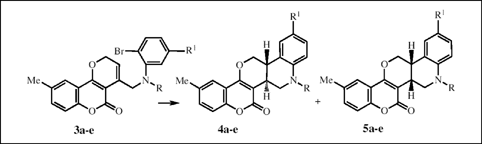
Regioselective synthesis of a number of coumarin-annulated pentacyclic heterocycles have been achieved by tri-n-butyltin hydride-mediated aryl radical cyclization. The products are formed as a mixture of cis- and trans- forms which were successfully separated by careful silica gel flash chromatography.