Article
Preparation of derivatives of 1-(2-pyrimidinyl)piperazine as potential antianxiety, antidepressant, and antipsychotic agents
Irwin Becker,
Irwin Becker
Department of Chemistry, Villanova University, Villanova, PA 19085
Search for more papers by this authorIrwin Becker,
Irwin Becker
Department of Chemistry, Villanova University, Villanova, PA 19085
Search for more papers by this authorAbstract
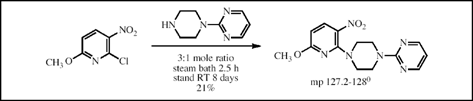
This paper describes the preparation of twenty-eight derivatives of 1-(2-pyrimidinyl)piperazine as potential antianxiety, antidepressant, and antipsychotic agents. In twenty-six of the preparations a chloro nitrogen heterocycle was caused to react with an excess of 1-(2-pyrimidinyl)piperazine in the absence of solvent. A specific example is given above.
References and Notes
- 1 Saudour, F.; Amara, D. A.; Dierich, A.; LeMeur, M.; Ramboz, S.; Segu, L.; Buhot, M-C.; Hen, R. Science, New Series 1994, 265, 1875.
- 2
Duman, R. S.;
Heninger, G. R.;
Nestler, E. J.
Archives of General Psychiatry
1997,
57,
597.
10.1001/archpsyc.1997.01830190015002 Google Scholar
- 3 Uphouse, L. Neuroscience and Biochemical Reviews 1997, 21, 679.
- 4 Meltzer, H. Y. Neuropsychopharmacology 1999, 21, 106 S.
- 5 Barnes, N. M.; Sharp, T. Neuropharmacology 1999, 38, 1083.
- 6 Moret, C.; Briley, M. Eur. J. Pharmacology 2000, 404, 1.
- 7 Gingrich, J. A.; Hen, R. Psychopharmacology (Berlin) 2001, 155, 1.
- 8 Manji, H. K.; Drevets, W. C.; Charney, D. S. Nature Medicine 2001, 7, 541.
- 9 Svenningssen, P.; Chergui, K.; Rachleff, I.; Flagjolet, M.; Zhana, X.; El Yacoubi, M.; Vaugeois, J. M.; Nomikos, G. S.; Greengard, P. Science 2006, 311, 77.
- 10 Mokrosz, J. M.; Strekowski, L.; Buszynska, B.; Harden, D. B.; Mokrosz, M. J.; Bajarski, A. J. Pharmazie 1994, 49, 801.
- 11 Matsumoto, K.; Hashimoto, S.; Minatogawa, H.; Munakata, M.; Otani, S. Chemistry Express 1990, 5, 473.
- 12 Matsumoto, K.; Hashimoto, S.; Toda, M.; Hashimoto, M.; Otani, S. Chemistry Express 1991, 6, 775.
- 13 Arranz, M. E.; Diaz, J. A.; Vega, S.; Campos-Toimil, M.; Orallo, F.; Cardelus, I.; Llenas, J.; Fernandez, A. G. Eur. J. Med. Chem. 2000, 35, 751.
- 14 Vega, S.; Arranz, M. E.; Aran, V. J. J. Heterocyclic Chem. 2005, 42, 763.
- 15 Regnier, G.; Canevari, R.; Le Douarec, J. C.; Laubie, M. Chimica Therapeutica 1972, 7, 192.
- 16 Campbell, S. F.; Plews, R. M. J. Med. Chem. 1987, 30, 1794.
- 17a Goldberg, H. L.; Finnerty, R. J. Am. J. Psychiatry 1979, 136, 1184. 17b Rickels, K. J. Clinical Psychiatry 1981, 42, 40. 17c Gammans, R. E.; Mayol, R. F.; LaBudde, J. A.; Gasten, G. P. Federation Proc. 1982, 41, 1335, abstract no. 6223. 17d Riblet, L. A.; Taylor, D. P.; Eison, M. S.; Stanton, H. C. J. Clinical Psychiatry 1982, 43, 11. 17e Newton, R. E.; Casten, G. P.; Alms, D. R.; Benes, C. O.; Marunycz, J. D. J. Clinical Psychiatry 1982, 43, 100. 17f Feighner, J. P.; Meredith, C. H.; Hendrickson, G. A. J. Clinical Psychiatry 1982, 43, 103. 17g Caccia, S.; Conti, I.; Vigano, G.; Garattini, S. Pharmacology 1986, 33, 46. 17h Schweizer, E. E.; Amsterdam, J.; Rickels, K.; Kaplan, M.; Droba, M. Psychopharmacology Bulletin 1986, 22, 183. 17i Gammans, R. E.; Mayol, R. F.; LaBudde, J. A. The American Journal of Medicine 1986, 80 (suppl. 3B), 41. 17j Dischino, D. D.; Covington, R. R.; Combs, C. M.; Gammans, R. E. J. Labelled Compounds and Pharmaceuticals 1987, 25, 359. 17k Amsterdam, J. D.; Berwish, N.; Potter, L.; Rickels, K. Current Therapeutic Research 1987, 41, 185. 17l Bianchi, G.; Caccia, S.; Della Vedova, F.; Garattini, S. Eur. J. Pharmacol. 1988, 151, 365. 17m Cott, J. M.; Kurtz, N. M.; Robinson, D. S.; Lancaster, S. P.; Copp, J. E. Psychopharmacology Bull. 1988, 24, 164. 17n Jajoo, H. K.; Mayol, R. F.; LaBudde, J. A.; Blair, I. A. Drug Metabolism and Disposition 1989, 17, 634. 17o Taylor, D. P. Ann. NY Academy of Sciences 1990, 600, 545. 17p Rausch, J. L.; Ruegg, R.; Moeller, F. G. Psychopharmacology Bull. 1990, 26, 169. 17q Heller, A. H.; Beneke, M.; Kuemmel, B.; Spencer, D.; Kurtz, N. M. Psychopharmacology Bull. 1990, 26, 219. 17r Robinson, D. S.; Rickels, K.; Feighner, J.; Fabre Jr., L. F.; Gammans, R. E.; Shrotriya, R. C.; Alms, D. R.; Andary, J. J.; Messina, M. E. J. Clinical Psychopharmacology 1990, 10 (3 suppl.), 67S–76S. 17s Jenkins, S. W.; Robinson, D. S.; Fabre Jr., L. F.; Andary, J. J.; Messina, M. E.; Reich, L. A. J. Clinical Psychopharmacology 1990, 10 (3 suppl.), 77S–85S. 17t Rickels, K.; Amsterdam, J. D.; Clary, C.; Puzzuoli, G.; Schweizer, E. E. J. Clin. Psychiatry 1991, 52, 34. 17u Pinder, R. M.; Weiringa, J. H. Medical Research Reviews 1993, 13, 259. 17v Kerns, E. H.; Volk, K. J.; Hail, M. E.; Whitney, J. L.; Rourick, R. A.; Klohr, S. E.; Leel, M. S., Paper originally presented at The 1996 International Symposia on Laboratory Atomation and Robotics (ISLAR 96). 17w Barradell, L. B.; Fitton, A. CNS Drugs 1996, 5, 147. 17x Fulton, B.; Brogden, R. N. CNS Drugs 1997, 7, 68.
- 18 Pinder, R. M. Psychopharmacology: Recent Advances and Future Prospects 1986, pp. 44–62.
- 19 Thurkauf, A.; Yuan, J.; Chen, X.; He, X. S.; Wasley, J. W. F.; Hutchinson, A.; Woodruff, K. H.; Meade, R.; Hoffman, D. C.; Donovan, H.; Jones-Hertzog, D. K. J. Med. Chem. 1997, 40. 1.
- 20 Tallman, J. F.; Primos, R. J.; Brodbeck, R.; Cornfield, L.; Meade, R.; Woodruff, K.; Ross, P.; Thurkauf, A.; Gallagher, J. J. of Pharmacology and Experimental Therapeutics 1997, 282, 1011.
- 21 Schaus, J. M.; Bymaster, F. B. Annual Reports in Medicinal Chemistry 1998, 33, 1.
- 22 We had prepared 4 earlier. The preparation of 4 is described in the paper by I. Becker J. Heterocyclic Chem. 2005, 42, 1289. The same preparative details are given in this paper.
- 23 I. Becker, PhD Dissertation, Temple University, Phila, PA, 1961, pp 105–107. Our experimental procedure was patterned after that described by Whitmore, F. C.; Mosher, H. S.; Adams, R. R.; Taylor, R. B.; Chapin, E. C.; Weisel, C.; Yanko, W. J. Am. Chem. Soc. 1944, 66, 725 and after procedures given by Bruson, H. A., Cyanoethylation in Organic Reactions R. Adams, ed., John Wiley and Sons, Inc., NY, 1949, Vol. 5, pages 109ff.
- 24 The structure of 29 was confirmed by a positive Beilstein test (C1 present), by CHN analyses, by an ir spectrum that indicated the presence of an aliphatic CN group in the molecule, and by a 1H nmr spectrum.
- 25 Baker, B. R.; Tanna, P. M. J. Org. Chem. 1965, 30, 2857.
- 26 Rosemeyer, H.; Seela, F. Heterocycles 1985, 23, 2669.
- 27 The preparation of 24 had been reported earlier by Regnier, G.; Canevari, R.; LeDouarec, J.-C.; Laubie, M. Chimie Therapeutique 1972, 7, 192. But specific experimental details for the preparation of 24 are not given in this paper. Instead, details are given for the preparation of a compound of analogous structure. Apparently, then, they prepared 24 by refluxing a mixture of 1-(2-pyrimidinyl)piperazine and 6-chloropurine (2:1 mol ratio) in dimethylformamide (normal bp 153°) for 7 h. Their product, recrystallized from dimethylformamide, had mp greater than 350°. They give no spectral data for their product. Our preparation of 24 differed significantly from that of Regnier and coworkers. In our preparation a mixture of 6.05 g (0.0369 mol) of 1-(2-pyrimidinyl)piperazine and 1.90 g (0.0123 mol) of 6-chloropurine (3:1 mol ratio of amine to 6-chloropurine) was heated on the steam bath for 3.5 h, let stand for 67 h, heated on the steam bath for 4 h more, and let stand for 45 h. Our crude product gave a negative Beilstein test (no C1). It was recrystallized from the solvent pair of 95% ethanol and water. Our once recrystallized product gave an acceptable CHN analysis, and gave a 1H nmr spectrum that confirmed its structure.
- 28 Our product 26, prepared by cyanoethylation of 24, gave an acceptable CHN analysis, gave an ir spectrum that indicated the presence of an aliphatic CN group in the molecule, and gave a 1H nmr spectrum that confirmed its structure.
- 29 SciFinder lists no references for either 27 or 28 but does list five commercial sources for 27 and one commercial source for 28.