Pathological spectrum of bile duct lesions from chronic bile duct injury to invasive cholangiocarcinoma corresponding to bile duct imaging findings of occupational cholangiocarcinoma
Abstract
Background
We aimed to identify the pathological characteristics of occupational cholangiocarcinoma.
Methods
We examined the location and distribution of the carcinomas: atypical epithelium including biliary intraepithelial neoplasia (BilIN) and intraductal papillary neoplasm of the bile duct (IPNB); and chronic bile duct injuries in operative or autopsy liver specimens from 16 patients. We examined the detailed pathological findings and diagnostic imaging of three patients. Immunohistochemical analysis using primary antibodies against γH2AX and S100P was performed.
Results
BilIN and chronic bile duct injury were observed in 16 patients, and IPNB or invasive IPNB was observed in 11 patients. BilIN, IPNB, and/or chronic bile duct injury were observed in almost all the large bile ducts. Regional dilatation of the bile ducts without tumor-induced obstruction revealed such pathological changes. Highly positive results for the γH2AX and S100P markers were noted in invasive carcinoma, BilIN, and IPNB, whereas positive results for γH2AX and negative results for S100P were noted in non-neoplastic biliary epithelium.
Conclusions
The carcinogenic process of occupational cholangiocarcinoma comprised chronic bile duct injury and DNA damage in almost all the large bile ducts, along with induction of precancerous lesions and development of invasive carcinoma. Such pathological findings reflected radiological changes on diagnostic imaging.
Introduction
An outbreak of cholangiocarcinoma in printing companies in Japan was recently reported 1-3, and such cholangiocarcinoma was first recognized as an occupational disease by the Japanese Ministry of Health, Labour and Welfare in 2013 4. By September 2015, 36 patients, including 17 patients at the printing company in Osaka 1, were diagnosed with occupational cholangiocarcinoma by the Ministry. It was reported that 1,2-dichloropropane (DCP) and dichloromethane (DCM) play an important role in the development of this type of cholangiocarcinoma 1-4. In June 2014, the International Agency for Research on Cancer decided to classify DCP as group 1 (carcinogenic to humans) and DCM as group 2A (probably carcinogenic to humans) 5. We previously reported that an increased serum concentration of gamma–glutamyl transpeptidase (γ-GTP) activity, the regional dilatation of the intrahepatic bile ducts without tumor-induced obstruction and biliary images similar to the appearance of primary sclerosing cholangitis (PSC), such as multiple strictures of the bile ducts with or without fusiform dilatation 6 on diagnostic imaging, papillary proliferating tumor, and the presence of precancerous or early cancerous lesions, such as biliary intraepithelial neoplasia (BilIN) and intraductal papillary neoplasm of the bile ducts (IPNB), and non-specific bile duct injuries, such as fibrosis, are characteristic of patients with occupational cholangiocarcinoma 1, 3, 7. However, the number of subjects was small, and an extensive pathological examination and comparison between pathological findings and radiological findings have not been performed in previous studies 1, 3, 7.
In this study, we evaluated the pathological features of 16 patients with occupational cholangiocarcinoma. In addition, for three of the 16 patients, whose detailed radiological findings and whole operative specimens for extensive pathological examination were available, we examined the radiological and pathological findings more closely. We also discuss the possible carcinogenic progression of occupational cholangiocarcinoma.
Subjects and methods
Subjects
The subjects in this study consisted of 16 men with occupational cholangiocarcinoma who were former and current workers at printing companies in Japan (Hokkaido, Miyagi, Osaka, and Fukuoka prefectures) and whose operative or autopsy specimens, including hepatic tissue, were available for pathological examination (Table 1). They were exposed to various types of chlorinated organic solvents, including DCP, DCM, and 1,1,1-trichloroethane (TCE). We evaluated the clinicopathological findings in the 16 patients using surgical specimens from 14 patients (patients no. 1, 2, 4–12, and 14–16) and autopsy specimens from two patients (patients no. 3 and 13). We also performed a detailed pathological examination of the whole resected liver, including immunohistochemical analysis corresponding to the bile duct imaging findings, while making anatomical charts of the large bile ducts in three patients (patients no. 10–12). “Large bile duct” is used as a collective term that refers to the common hepatic duct, the left or right hepatic duct, or to the first to third branches of the intrahepatic bile duct 8.
| Patients | Age | Gender | Organic solvents | Location and type of cholangiocarcinoma | Treatments | Specimens | BilIN-2/3 | IPNB/invasive IPNB | Chronic bile duct injury |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 34 | M | DCP, DCM, TCE | ICC, mass-forming | Extended rt. hepatectomy, resection of extrahepatic bile duct | Surgery | Yes | Yes | Yes |
| 2 | 34 | M | DCP, DCM, TCE | ICC, mass-forming | Rt. trisegmentectomy | Surgery | Yes | Yes | Yes |
| 3 | 25 | M | DCP | ICC, mass-forming | Chemotherapy | Autopsy | Yes | ND | Yes |
| 4 | 35 | M | DCP, DCM, TCE | ECC (perihilar), papillary | Extended rt. hepatectomy, resection of extrahepatic bile duct | Surgery | Yes | Yes | Yes |
| 5 | 40 | M | DCP, DCM, TCE | ICC, mass-forming ECC (distal), papillary | Rt. Hepatectomy, Pancreaticoduodenectom | Surgery | Yes | Yes | Yes |
| 6 | 38 | M | DCP, DCM | ICC, mass-forming | Segmentectomy 8 | Surgery | Yes | ND | Yes |
| 7 | 40 | M | DCP, DCM, TCE | ICC, intraductal growth | Extended lt. hepatectomy | Surgery | Yes | Yes | Yes |
| 8 | 31 | M | DCP | ECC (perihilar), papillary | Extended rt. hepatectomy, resection of extrahepatic bile duct | Surgery | Yes | Yes | Yes |
| 9 | 39 | M | DCP | ICC, intraductal growth ECC (perihilar), papillary | Lt. hepatectomy, resection of extrahepatic bile duct | Surgery | Yes | Yes | Yes |
| 10 | 39 | M | DCP | ICC, intraductal growth | Lt. hepatectomy, Segmentectomy 7 | Surgery | Yes | Yes | Yes |
| 11 | 31 | M | DCP | ICC, mass-forming | Rt. hepatectomy, resection of extrahepatic bile duct | Surgery | Yes | Yes | Yes |
| 12 | 34 | M | DCP | ICC, intraductal growth | Extended lt. hepatectomy | Surgery | Yes | Yes | Yes |
| 13 | 37 | M | DCP, DCMa, TCE | ICC, mass-forming | Chemotherapy | Autopsy | Yes | Yes | Yes |
| 14 | 42 | M | DCP, DCMa, TCE | ECC (perihilar), scirrhous constricting | Extended rt. hepatectomy withpreoperative chemotherapy and radiotherapy | Surgery | Yes | No | Yes |
| 15 | 57 | M | DCP, DCM | ICC, mass-forming | Extended rt. hepatectomy, resection of extrahepatic bile duct | Surgery | Yes | No | Yes |
| 16 | 47 | M | DCP, DCM | ICC, mass-forming | Rt. trisegmentectomy | Surgery | Yes | ND | Yes |
- BilIN biliary intraepithelial neoplasia, DCM dichloromethan, DCP 1,2-dichloropropane, ECC extrahepatic cholangiocarcinoma, ICC intrahepatic cholangiocarcinoma, IPNB intraductal papillary neoplasm of the bile duct, invasive IPNB IPNB with an associated invasive carcinoma, ND not determined because of small amount of non-cancerous hepatic tissue, TCE 1,1,1-trichloroethane.
- a The amount of DCM used was small
This study was approved by the ethics committee of Osaka City University, and all subjects or their legally authorized representatives (for deceased patients) provided written informed consent. This multicenter occupational cholangiocarcinoma study group consisted of investigators at 15 institutes.
Pathological examination
We evaluated the clinicopathological characteristics, including the presence of BilIN or IPNB, in non-cancerous hepatic tissue, and the presence of chronic bile duct injury in the 16 patients. The pathological findings were evaluated by pathologists (Y.N. and Y.S.) according to the World Health Organization classifications for intrahepatic cholangiocarcinoma 9. Pre- or early neoplastic lesions of the bile ducts were classified as BilIN or IPNB. BilIN lesions were histologically classified according to their cellular and structural features as BilIN-1 (mild atypia), BilIN-2 (moderate atypia), or BilIN-3 (severe atypia corresponding to in situ carcinoma). In this study, we mainly surveyed BilIN-2 and BilIN-3 lesions because it was unclear whether BilIN-1 lesions included reactive hyperplastic changes. “Chronic bile duct injury” is a collective term that refers to various combinations of duct injuries such as epithelial damage, fibrosis of the bile duct wall and periductal tissue, and chronic inflammatory cell infiltration.
We evaluated the detailed pathological findings of the whole resected liver while making anatomical charts of the large bile ducts in three patients (patients no. 10–12) as follows. Left hepatectomy and excision of segment 7 were performed in patient 10. Right hepatectomy and resection of the extrahepatic bile duct with hepaticojejunostomy and reconstruction were performed in patient 11. Extended left hepatectomy was performed in patient 12. To enable the extensive pathological examination of all the operative specimens, 56 histological sections (5-mm each) were obtained from the specimen of patient 10, 85 sections from the specimen of patient 11, and 124 sections from the specimen of patient 12. After macroscopic observation of the specimens, the sections were cut, embedded in paraffin, and stained with hematoxylin-eosin (HE). Observed lesions including the main tumor(s), precancerous lesions such as BilIN-2/3 and IPNB, and chronic bile duct injury on HE-stained specimens were cross-referenced with the anatomical charts of the bile ducts. The pathological findings were mapped to evaluate the correlation between the diagnostic imaging and pathological findings.
Immunohistochemistry
Immunological staining was performed using primary antibodies against S100P (1:100 rabbit monoclonal, Epitomics) and γH2AX (1:100 rabbit monoclonal; Novus Biologicals, Littleton, CO, USA) to evaluate neoplastic changes and DNA injury locations, respectively, in three patients (patients no. 10–12). After deparaffinization, antigen retrieval was performed by autoclaving the sections in citrate buffer 10 mmol/l (pH 6.0). The sections were then immersed in 0.3% hydrogen peroxidase in purified water for 20 min at room temperature to block endogenous peroxidase activity. After pretreatment with blocking serum (Blocking One; NACALAI TESQUE), the sections were incubated overnight at 4°C with each of the primary antibodies. The sections were then incubated with a secondary antibody conjugated to peroxidase-labeled polymer using the HISTOFINE system (Nichilei, Tokyo, Japan). Color development was performed using 3,3′-diaminobenzidine tetrahydrochloride, and the sections were lightly counterstained with hematoxylin. A semiquantitative analysis of the immunostained sections was performed. According to the mapping charts of invasive carcinoma, BilIN-2/3, IPNB, peribiliary gland, and chronic bile duct injuries were observed in fields at × 200 magnification, and the area of highest labeling of γH2AX and S100P nuclear expression were selected for each focus in the sections. The proportion of stained cells was evaluated as follows: – (negative); + (≤20%); and ++ (>20%).
Anatomical charts of the bile duct
Dynamic computed tomography (CT), ultrasonography (US), magnetic resonance cholangiopancreatography (MRCP), and endoscopic retrograde cholangiopancreatography (ERCP) studies were performed in these three patients. Anatomical charts of the large bile ducts were created to depict the pathological changes of the large bile ducts by reference to these diagnostic images in each case. The spectrum of biliary tract lesions including stricture or obstruction of the bile duct, regional dilatation of the intrahepatic bile ducts without tumor-induced obstruction were evaluated on these diagnostic images.
The liver anatomy and operative methods were classified according to Brisbane 2000 terminology 10.
Results
Clinicopathological findings of 16 patients
The age of the patients at diagnosis ranged from 25 to 57 years (mean, 37 years), and all patients were men (Table 1). Of the 16 patients, seven patients (patients no. 1, 2, 4, 5, 7, 13, and 14) had been exposed to DCP, DCM, and TCE; three patients (patients no. 6, 15, and 16) had been exposed to DCP and DCM; and six patients (patients no. 3, and 8–12) had been exposed to only DCP. The main tumor was classified as intrahepatic cholangiocarcinoma in 11 patients, and extrahepatic cholangiocarcinoma (perihilar) in three patients. One patient was diagnosed as having intrahepatic and extrahepatic (perihilar) cholangiocarcinoma, and another patient was diagnosed as having intrahepatic and extrahepatic (distal side) cholangiocarcinoma.
The histopathological examination showed that the main tumor in the 16 patients was adenocarcinoma. BilIN-2/3 lesions and chronic bile duct injury were observed in all 16 patients (Table 1). IPNB, including IPNB with an associated invasive carcinoma (invasive IPNB) and some mucus production, was observed in 11 patients (patients no. 1, 2, 4, 5, and 7–13). In three patients (patients no. 3, 6, and 16), the presence of IPNB could not be evaluated sufficiently because of the presence of small amounts of non-cancerous hepatic tissue. In four patients in whom the whole resected liver (patients no. 10–12) or the whole liver by autopsy (patients no. 13) were available for pathological examination, BilIN-2/3, IPNB, and chronic bile duct injury were observed in almost all of the large bile ducts.
Extensive pathological examination of the whole resected specimens
Patient 10
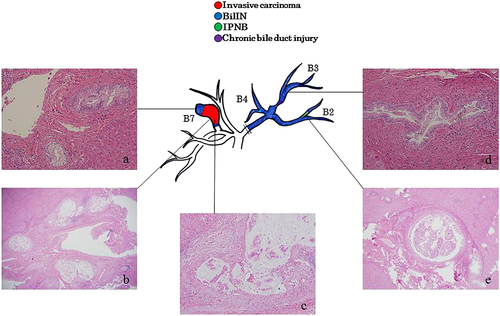
On the preoperative MRCP (Fig. 1a), regional dilatation of the peripheral bile ducts without tumor-induced obstruction was observed in segment 7 (B7). Multiple strictures and regional dilatation of the bile ducts in the left lobe were also seen. Macroscopic examination of the operative specimens showed the dilatation of B7 (Fig. 1b) and thickening of Glisson's sheath in the left lobe (Fig. 1c), corresponding to the MRCP findings. On the histopathological examination, well differentiated papillary adenocarcinoma with mucus-producing eosinophilic cytoplasm (intraductal growth type) was observed in the B7 dilatation (Fig. 2b,c), and it was diagnosed to be an oncocytic type invasive IPNB with minimal invasion. BilIN2/3 lesions with different morphology from that of the main lesion were observed in the peripheral portions of B7 (Fig. 2a). In the left lobe, BilIN-2/3 was observed throughout the bile ducts, which were not abnormal on the preoperative imaging (Fig. 2d). IPNB was observed in the peripheral bile ducts in segment 2 (B2), corresponding to the regional dilatation of the bile ducts seen on the preoperative image (Fig. 2e). Chronic multifocal bile duct injuries were found throughout the bile ducts.
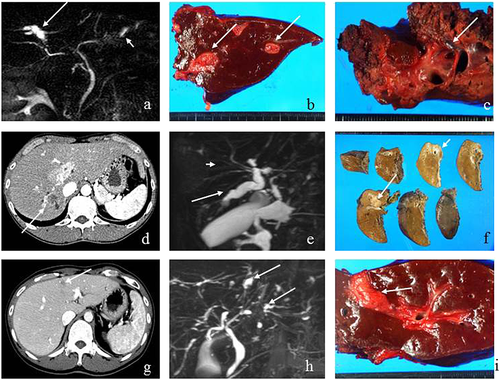
Patient 11
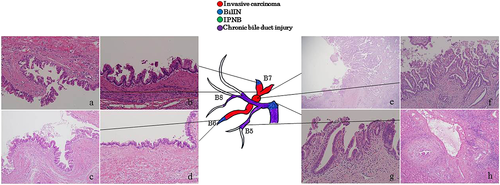
A space-occupying lesion was observed in segment 6 on the preoperative CT (Fig. 1d). Dilated bile ducts in the posterior segment (peripheral side of the tumor) as well as multiple strictures and dilatation of the bile ducts in the anterior segment were observed on MRCP and ERCP (Fig. 1e). Macroscopic examination of the operative specimens showed a tumor in segment 6 (mass-forming type) and dilatation of the bile ducts (Fig. 1f). Histopathologically, a space-occupying lesion on preoperative diagnostic imaging was an oncocytic type of invasive adenocarcinoma with micropapillary lesions on the luminal sides and extramural invasion and periductal infiltration to the posterior branch (Fig. 3a,e,f). BilIN-2/3 lesions were observed in the peripheral portions of the bile ducts in segments 6 and 7 (B6, B7) (Fig. 3b). In the peripheral parts of B6, a mixture of atypical epithelium with the same form as the main tumor and atypical epithelium with a different form (Fig. 3c,d) were observed. BilIN-3 lesions were observed in the hepatic duct, which was stenotic on the preoperative diagnostic imaging (Fig. 3g). Chronic bile duct injuries were observed in the bile ducts of the anterior segment and hepatic duct that corresponded to the stricture and dilatation of the bile duct on preoperative diagnostic imaging (Fig. 3h).
Patient 12
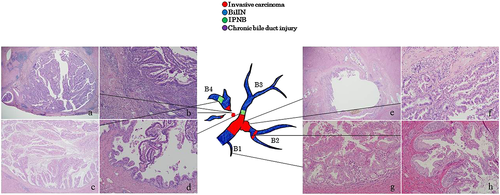
Preoperative CT showed tumors in the bile ducts in segment 4 (B4), MRCP showed dilatation in the peripheral and proximal sides of B4 (Fig. 1g,h). The macroscopic findings of the operative specimens showed a tumorous lesion on the proximal side of B4 (Fig. 1i) that was further identified as a papillary adenocarcinoma with intraductal growth (intrductal growth type). Histopathologically, the carcinoma cells showed acidophilic cytoplasm, and the tumor was classified as an oncocytic type invasive IPNB (Fig. 4a,b). The tumor invaded outside the wall and the atypical epithelium spread to the proximal side of the bile ducts in segment 2 (B2), corresponding to the bile duct dilatation seen on the preoperative diagnostic imaging (Fig. 4e,f). Moreover, IPNB lesions differed from the main tumor observed in the peripheral portions of B4 and B3 (Fig. 4c,d). BilIN-2/3 lesions with gastric metaplasia were observed throughout the bile ducts (Fig. 4g,h). Chronic bile duct injuries were detected in various sites of the bile ducts, although these ducts appeared normal on preoperative diagnostic imaging.
Immunohistochemical analysis
In three patients (patients no. 10–12), positive expressions of γH2AX and S100P were detected in almost all portions of the invasive carcinoma, BilIN-2/3, and IPNB (Fig. 5a–f, Table 2) in the large bile ducts. Weakly positive expression was also seen at the peribiliary glands (Fig. 5 g,h). In two patients (patients no. 10 and 11) who could evaluated for non-neoplastic biliary epithelium, although γH2AX expression was also detected within the non-neoplastic biliary epithelium similar to BilIN, IPNB, and invasive carcinoma, S100P expression was absent or relatively weak in non-neoplastic epithelium (Fig. 5i,j). The specific expression of neither γH2AX nor S100P was detected in the hepatocytes of any of these patients.
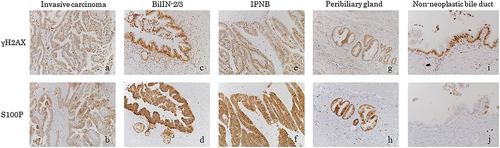
| Enzyme | Patients | Invasive carcinoma | BilIN-2/3 | IPNB | Peribiliary gland | Non-neoplastic bile duct |
|---|---|---|---|---|---|---|
| γH2AX | 10 | ++ | ++ | ++ | + | + |
| 11 | ++ | ++ | ++ | ++ | + | |
| 12 | ++ | ++ | ++ | + | ND | |
| S100P | 10 | ++ | ++ | ++ | + | − |
| 11 | ++ | ++ | ++ | + | − | |
| 12 | ++ | ++ | ++ | + | ND |
- BilIN biliary intraepithelial neoplasia, IPNB intraductal papillary neoplasm of the bile duct, ND not determined because there was not non-neoplastic epithelium in large bile ducts.
- –, negative expression; +, positive expression but not exceeding 20%; ++, positive expression more than 20%
Discussion
The findings obtained in this study of the 16 patients of occupational cholangiocarcinoma are summarized as follows: (1) A spectrum of pathological changes such as chronic bile duct injury and early neoplastic and pre-invasive lesions such as BilIN and/or IPNB and invasive cholangiocarcinoma was observed in the biliary tree in all 16 patients. Such pathological findings were observed in almost all of the large bile ducts with or without sclerotic changes through extensive pathological observation while referring to biliary imaging in three patients. (2) This spectrum of biliary tract lesions reflected biliary tract images such as PSC-like changes including regional bile duct dilatation without tumor-induced obstruction in these patients. (3) Aberrant expression of S100P was seen in pre-invasive neoplastic lesions in addition to invasive cholangiocarcinoma, and DNA damage was even found in chronic bile duct injuries in addition to neoplastic biliary lesions.
BilIN and IPNB are currently regarded as precancerous or early cancerous lesions as per the World Health Organization's classifications of cholangiocarcinoma 9, and are considered to be involved in the multistep carcinogenesis of cholangiocarcinoma in patients with hepatolithiasis 11-13. Papillary or invasive carcinoma, precancerous or early cancerous lesions such as BilIN and IPNB, and non-specific bile duct injuries such as fibrosis are reportedly pathological characteristics of occupational cholangiocarcinoma 1, 3, 7. In a previous study 11, BilIN-2/3 was observed in nine of 19 patients with cholangiocarcinoma associated with hepatolithiasis, and IPNB was observed in 10 of the 19 patients. In addition, BilIN-2/3 was observed in 24 of 55 patients with hepatolithiasis without cholangiocarcinoma, and IPNB was observed in nine of 55 of these patients. Such lesions were observed in the large bile ducts containing stones and in the adjacent bile ducts 11. Multistep carcinogenesis from BilIN was also suggested in PSC 14. Lewis et al. reported that BilIN-2/3 lesions were observed in 50% of patients with end-stage PSC who underwent liver transplantation 15. In this study, BilIN lesions were observed in all 16 patients, whereas occupational cholangiocarcinoma and IPNB or invasive IPNB were observed in 11 of the 16 patients; however, it was difficult to examine pathological findings because of the presence of insufficient materials from three patients (patients no. 3, 6, and 16). In addition, BilIN and IPNB were observed in almost all of the large bile ducts in the whole liver obtained by autopsy (patients no. 13) as well as the whole resected liver (patients no. 10–12). Thus, the high frequency of BilIN and/or IPNB and the wide distribution of such lesions were characteristic of the patients with occupational cholangiocarcinoma in comparison with patients with hepatolithiasis or PSC.
Intrahepatic cholangiocarcinomas in patients 10 and 12 were classified as intraductal growth type and the carcinoma in patient 11 was classified as a mass-forming type. Intrahepatic cholangiocarcinoma is classified into the perihilar large bile duct type and the peripheral small bile duct type 16, 17. In the perihilar large bile duct type, chronic biliary inflammation caused by PSC, hepatolithiasis, and liver flukes may induce BilIN followed by periductal infiltrating carcinoma as well as the mass-forming type cholangiocarcinoma and IPNB followed by intraductal papillary tumor growth (intraductal growth type cholangiocarcinoma and invasive IPNB). In this study, BilIN was observed in all 16 patients and IPNB was observed in 11 of the 16 patients, which indicates that the patients with occupational cholangiocarcinoma could either follow the BilIN or IPNB pathway. The extensive pathological examination in three patients (patients no. 10–12) showed that although the three patients had the potential for following either of these pathways, the carcinogenic process in the pathway involving BilIN was primarily followed in patient 11, whereas the carcinogenic process in the pathway involving IPNB was primarily followed in patients 10 and 12.
S100P is a 95-amino-acid protein that has been shown to mediate tumor growth, metastasis, and invasion through the binding of Ca2+ ions and receptors for advanced glycation end products 18. Increased S100P levels have been observed in carcinoma of the pancreas, biliary tract, lung, breast, and ovary 19. In addition, the previous reports showed that S100P was aberrantly expressed in perihilar cholangiocarcinoma 20-22, and that it was detected in BilIN-2/3 as well as invasive carcinoma in patients with hepatolithiasis 21, 22. In this study, S100P was aberrantly and strongly positive in BilIN-2/3 lesions and IPNB, while invasive carcinoma was also positive to S100P. However, in the non-neoplastic epithelium expressing γH2AX (see below), S100P expression was relatively weak or absent.
One of the earliest steps in the cellular response to DNA double-strand breaks is the phosphorylation of histone H2AX at serine 139, the γ-phosphorylation site, which results in γH2AX 23. On immunohistochemical analyses, γH2AX was highly expressed in many premalignant lesions, cancer cells, and solid tumors 24-28. In our previous study, γH2AX expression was observed in the foci of BilIN, IPNB, and/or invasive cholangiocarcinoma in seven of 16 patients with cholangiocarcinoma associated with hepatolithiasis 29. In this study, γH2AX expression was observed in the foci of BilIN, IPNB, and invasive carcinoma in all of the three patients (patients no. 10–12) with occupational cholangiocarcinoma. In addition, in two patients (patients no. 10 and 11) who could be evaluated for non-neoplastic biliary epithelium, γH2AX expression was also detected within the non-neoplastic biliary epithelium similar to BilIN, IPNB, and invasive carcinoma. However, in non-neoplastic epithelium positively expressing γH2AX, S100P expression was absent or relatively weak, indicating that precancerous or cancerous lesions developed in the bile ducts affected by DNA injury. In addition, one previous study reported that non-neoplastic biliary epithelial cells of the large bile duct and peribiliary glands were negative for γH2AX in patients with cholangiocarcinoma associated with hepatolithiasis 29. The findings in the present study and previous study 29 indicate that DCP or its metabolites might induce DNA damage and neoplastic changes in almost all of the large bile ducts, and that the frequent expression of γH2AX is another characteristic of occupational cholangiocarcinoma, in comparison with cholangiocarcinoma associated with hepatolithiasis.
In this study, the biliary tree was extensively examined by using several histological sections from whole liver specimens and through the use of biliary tract images. We observed that the regional dilatation of intrahepatic bile ducts as well as bile duct sclerosis with or without multiple strictures similar to PSC corresponded to invasive cholangiocarcinoma, including papillary adenocarcinoma (invasive IPNB), precancerous or early cancerous lesions such as BilIN and IPNB, and chronic bile duct injury with fibrosis. In patients 10 and 11, a PSC-like appearance on the diagnostic images and changes in laboratory test results were observed several years before the diagnosis of cholangiocarcinoma was made 7. Thus, the identification of such changes on diagnostic imaging can be useful for screening and surveillance for occupational cholangiocarcinoma 30.
It appears plausible that chlorinated organic solvents (DCP and DCM) or their products induce chronic bile duct injury with DNA damage, gradually followed by precancerous lesions and invasive carcinoma—this is indicative of the multistep carcinogenesis process in occupational cholangiocarcinoma. The wide distribution of biliary epithelium with DNA injury and pre- or early cancerous lesions expressing S100P indicates that the various sites of the bile ducts of patients exposed to chlorinated organic solvents have malignant potential, and may eventually develop multifocal carcinogenesis. Although studies concerning occupational cholangiocarcinoma are rare because the number of patients with occupational cholangiocarcinoma is very small and the materials for the studies including resected specimens are limited, further analyses are necessary to understand the detailed mechanism of the development of occupational cholangiocarcinoma.
In conclusion, extensive pathological observation of the biliary tree of the surgically resected livers of patients with occupational cholangiocarcinoma showed a unique spectrum of biliary lesions including chronic bile duct injury, proliferative changes, pre-invasive neoplastic lesions, and invasive cholangiocarcinoma, which reflects the regional dilatation of the intrahepatic bile ducts and PSC-like changes seen on biliary tract imaging. Furthermore, a carcinogenic process consisting of chronic bile duct injury with DNA damage that occurred within almost all of the large bile ducts, the induction of pre- or early cancerous lesions such as BilIN and IPNB, and the development of invasive carcinoma is proposed. More studies are mandatory to evaluate the chronic biliary and DNA damage as well as the progression to neoplastic lesions.
Acknowledgments
This study was supported in part by Health and Labor Sciences Research Grants for Research on Occupational Safety and Health (epidemiological and cause-investigated study of cholangiocarcinoma in printing company workers) and by Industrial Disease Clinical Research Grants (establishment of diagnostic methods for occupational cholangiocarcinoma; 14040101-01). This work was also supported in part by the Japan Society for the Promotion of Science KAKENHI Grant Number 26245678 (clinicopathological and molecular biological analysis of carcinogenesis of intrahepatic cholangiocarcinoma by chemicals). We than Drs S. Marubashi and M. Kaji for their assistance with data collection.
Conflict of interest
None declared.
Author contributions
Study design: MK, SK, and YN designed the study. Acquisition of data: MK, SK, YN, Y. Sato, S. Takemura, S. Tanaka, GH, TI, HT, T. Yamada, SN, AA, MF, Y. Sugawara, T. Yamamoto, MA, KN, MU, TM, KT, and KS. Pathological aspect of the study: YN and Y. Sato analyzed pathological findings. Data analysis: MK, SK, YN, Y. Sato, and TS. Manuscript drafted by MK, SK, YN, and TS. All authors reviewed the manuscript.




